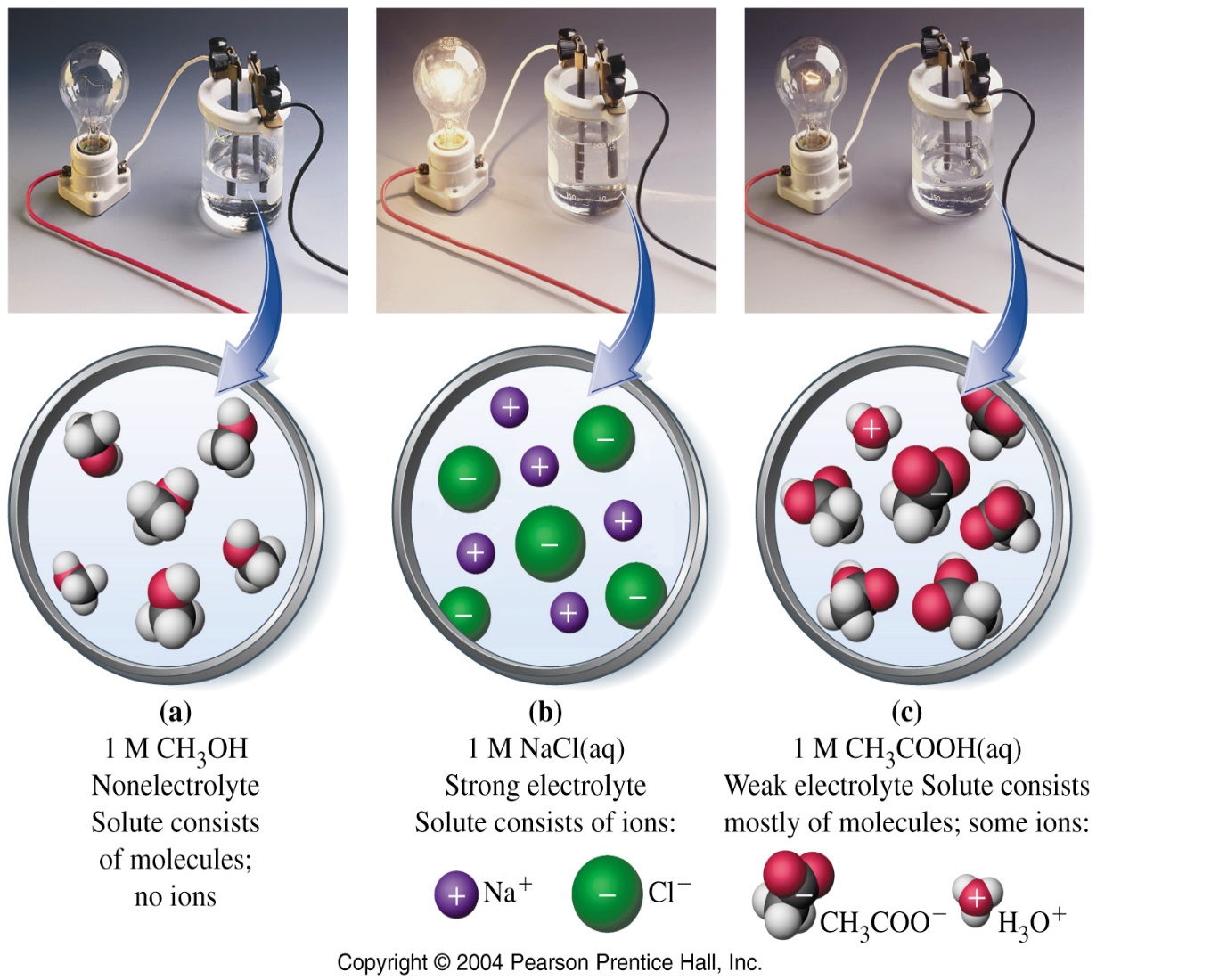
Examples Of Electrolyte Compounds . Electrolyte levels are often used to help. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Electrolytes are electrically charged compounds that are essential to the cells in your body. And some, such as silver iodide, are electrolytes even in the solid state. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. Most compounds that contain nitrogen are weak electrolytes. Weak electrolytes include weak acids, weak bases, and a variety of other compounds.
from www.w3schools.blog
Most compounds that contain nitrogen are weak electrolytes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Electrolyte levels are often used to help. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. And some, such as silver iodide, are electrolytes even in the solid state. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Electrolytes are electrically charged compounds that are essential to the cells in your body.
Strong and weak electrolytes W3schools
Examples Of Electrolyte Compounds Most compounds that contain nitrogen are weak electrolytes. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Electrolyte levels are often used to help. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. And some, such as silver iodide, are electrolytes even in the solid state. Electrolytes are electrically charged compounds that are essential to the cells in your body. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Most compounds that contain nitrogen are weak electrolytes.
From www.coursehero.com
[Solved] Which of these compounds is a strong electrolyte? A. CH3 COOH Examples Of Electrolyte Compounds The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). Electrolytes are electrically charged compounds. Examples Of Electrolyte Compounds.
From exokypmcv.blob.core.windows.net
Homemade Oral Electrolyte Solution at Dorothy Kelley blog Examples Of Electrolyte Compounds An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Most compounds that contain nitrogen are weak electrolytes. And some, such as silver iodide, are electrolytes even in the solid state. Electrolytes are electrically charged compounds that are essential. Examples Of Electrolyte Compounds.
From www.slideserve.com
PPT Aqueoussolution Reactions PowerPoint Presentation ID159152 Examples Of Electrolyte Compounds Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents. Examples Of Electrolyte Compounds.
From www.slideserve.com
PPT Lecture 7. Electrolytes. Reactions in Aqueous Solutions Examples Of Electrolyte Compounds Electrolytes are electrically charged compounds that are essential to the cells in your body. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. And some, such as silver iodide, are electrolytes even in the solid state. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and. Examples Of Electrolyte Compounds.
From www.slideserve.com
PPT Chemical reactions PowerPoint Presentation, free download ID Examples Of Electrolyte Compounds Electrolyte levels are often used to help. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. And some, such as silver iodide, are electrolytes even in the solid state. Weak electrolytes include weak acids, weak bases, and. Examples Of Electrolyte Compounds.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL Examples Of Electrolyte Compounds Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. And some, such as silver iodide, are electrolytes even in the solid state. Electrolytes are chemical compounds in the form of minerals that serve several. Examples Of Electrolyte Compounds.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Examples Of Electrolyte Compounds An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Electrolytes are electrically charged compounds that are essential to the cells in your body. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in. Examples Of Electrolyte Compounds.
From exylrahrq.blob.core.windows.net
What Is The Electrolyte Test at Robin Stutzman blog Examples Of Electrolyte Compounds And some, such as silver iodide, are electrolytes even in the solid state. Electrolytes are electrically charged compounds that are essential to the cells in your body. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and. Examples Of Electrolyte Compounds.
From www.slideserve.com
PPT Post Lab Electrolytes PowerPoint Presentation, free download Examples Of Electrolyte Compounds The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Electrolyte levels are often used to help. Common examples of electrolytes include sodium. Examples Of Electrolyte Compounds.
From www.pinterest.com
What is Electrolysis and Electrolyte with examples. Teaching Examples Of Electrolyte Compounds Electrolyte levels are often used to help. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Electrolytes are electrically charged compounds that are essential to the cells in your body. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium. Examples Of Electrolyte Compounds.
From www.slideserve.com
PPT II. Types of Electrolytes PowerPoint Presentation, free download Examples Of Electrolyte Compounds The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. And some, such as silver iodide, are electrolytes even in the solid state. Many salts, such as sodium chloride, behave as electrolytes when melted in the. Examples Of Electrolyte Compounds.
From www.slideserve.com
PPT Water, Electrolytes, and Solutions PowerPoint Presentation, free Examples Of Electrolyte Compounds Electrolytes are electrically charged compounds that are essential to the cells in your body. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such. Examples Of Electrolyte Compounds.
From www.youtube.com
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes Examples Of Electrolyte Compounds And some, such as silver iodide, are electrolytes even in the solid state. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Electrolyte levels are often used to help. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Most compounds that contain nitrogen are weak. Examples Of Electrolyte Compounds.
From www.animalia-life.club
Electrolyte Chemistry Examples Of Electrolyte Compounds The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Most compounds that contain nitrogen are weak electrolytes. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Electrolytes are electrically charged compounds that are essential to the cells in your body. Electrolyte levels are often used. Examples Of Electrolyte Compounds.
From www.osmosis.org
Overview of Electrolyte Balance Osmosis Video Library Examples Of Electrolyte Compounds Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). And some, such as silver iodide, are electrolytes even in the solid state. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Most compounds that contain nitrogen are weak electrolytes. Electrolyte. Examples Of Electrolyte Compounds.
From www.pinterest.com
Electrolytes vs Nonelectrolytes Secondary science, Brain book, Chemistry Examples Of Electrolyte Compounds Most compounds that contain nitrogen are weak electrolytes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Electrolyte levels are often used to help. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. An ionic compound for example, sodium chloride dissolved in water is called. Examples Of Electrolyte Compounds.
From pandai.me
Electrolytic Cell Examples Of Electrolyte Compounds Electrolytes are electrically charged compounds that are essential to the cells in your body. Most compounds that contain nitrogen are weak electrolytes. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. And some, such as silver. Examples Of Electrolyte Compounds.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Examples Of Electrolyte Compounds Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). Electrolytes are electrically charged compounds that are essential to the cells in your body. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Electrolytes are chemical compounds in the form of minerals that. Examples Of Electrolyte Compounds.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Examples Of Electrolyte Compounds And some, such as silver iodide, are electrolytes even in the solid state. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Weak electrolytes include weak acids, weak bases, and a variety of. Examples Of Electrolyte Compounds.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps Examples Of Electrolyte Compounds Electrolyte levels are often used to help. And some, such as silver iodide, are electrolytes even in the solid state. Most compounds that contain nitrogen are weak electrolytes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Electrolytes are chemical compounds in the form of minerals that serve several. Examples Of Electrolyte Compounds.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Examples Of Electrolyte Compounds Most compounds that contain nitrogen are weak electrolytes. Electrolyte levels are often used to help. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). Weak electrolytes include weak acids, weak bases, and a variety. Examples Of Electrolyte Compounds.
From www.pinterest.com
To Identify Given Solutions as Electrolyte and Non Electrolyte NEB Examples Of Electrolyte Compounds Electrolytes are electrically charged compounds that are essential to the cells in your body. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; And some, such as silver iodide, are electrolytes even in the solid state. An ionic. Examples Of Electrolyte Compounds.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Examples Of Electrolyte Compounds Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. Electrolytes are electrically charged compounds that are essential to the cells in your body. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and. Examples Of Electrolyte Compounds.
From www.numerade.com
SOLVED4) Which compounds are strong (electrolyte) acids and weak Examples Of Electrolyte Compounds Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Electrolytes are electrically charged compounds that are essential to the cells in your body. And some, such as silver iodide, are electrolytes even in the solid state. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium. Examples Of Electrolyte Compounds.
From www.w3schools.blog
Strong and weak electrolytes W3schools Examples Of Electrolyte Compounds Electrolytes are electrically charged compounds that are essential to the cells in your body. And some, such as silver iodide, are electrolytes even in the solid state. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Electrolyte levels are often used to help. An ionic compound for example, sodium chloride dissolved in water is called an. Examples Of Electrolyte Compounds.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Examples Of Electrolyte Compounds And some, such as silver iodide, are electrolytes even in the solid state. Most compounds that contain nitrogen are weak electrolytes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. Common examples of electrolytes include. Examples Of Electrolyte Compounds.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Examples Of Electrolyte Compounds The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Most compounds that contain nitrogen are weak electrolytes. And some,. Examples Of Electrolyte Compounds.
From www.thoughtco.com
Strong Electrolyte Definition and Examples Examples Of Electrolyte Compounds Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). Most compounds that contain nitrogen are weak electrolytes. And some, such as silver iodide, are electrolytes even in the solid state. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Electrolytes are chemical compounds in the form of. Examples Of Electrolyte Compounds.
From www.animalia-life.club
Electrolyte Chemistry Examples Of Electrolyte Compounds Most compounds that contain nitrogen are weak electrolytes. Electrolytes are electrically charged compounds that are essential to the cells in your body. Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl),. Examples Of Electrolyte Compounds.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Examples Of Electrolyte Compounds Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Electrolytes are electrically charged compounds that are essential to the cells in your body. Electrolyte levels are often used to help. Most compounds that contain nitrogen are weak electrolytes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in. Examples Of Electrolyte Compounds.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Examples Of Electrolyte Compounds Electrolytes are electrically charged compounds that are essential to the cells in your body. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; And some, such as silver iodide, are electrolytes even in the solid state. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. An ionic compound. Examples Of Electrolyte Compounds.
From dxolcsxuo.blob.core.windows.net
What Are The Normal Electrolyte Levels at Casey Cornell blog Examples Of Electrolyte Compounds Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Most compounds that contain nitrogen are weak electrolytes. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. Examples Of Electrolyte Compounds.
From studiousguy.com
9 Electrolyte Examples in Daily Life StudiousGuy Examples Of Electrolyte Compounds Most compounds that contain nitrogen are weak electrolytes. And some, such as silver iodide, are electrolytes even in the solid state. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)), and sodium bicarbonate (\(nahco_3\)). Electrolytes are electrically charged. Examples Of Electrolyte Compounds.
From www.bigstockphoto.com
Electrolyte Image & Photo (Free Trial) Bigstock Examples Of Electrolyte Compounds Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Electrolyte levels are often used to help. Most compounds that contain nitrogen are weak electrolytes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. An ionic compound for example, sodium chloride dissolved in water is called. Examples Of Electrolyte Compounds.
From pediaa.com
Difference Between Electrolytes and Nonelectrolytes Definition Examples Of Electrolyte Compounds Weak electrolytes include weak acids, weak bases, and a variety of other compounds. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. And some, such as silver iodide, are electrolytes even in the solid state. Common examples of electrolytes include sodium chloride (nacl), potassium chloride (kcl), calcium chloride (\(cacl_2\)),. Examples Of Electrolyte Compounds.