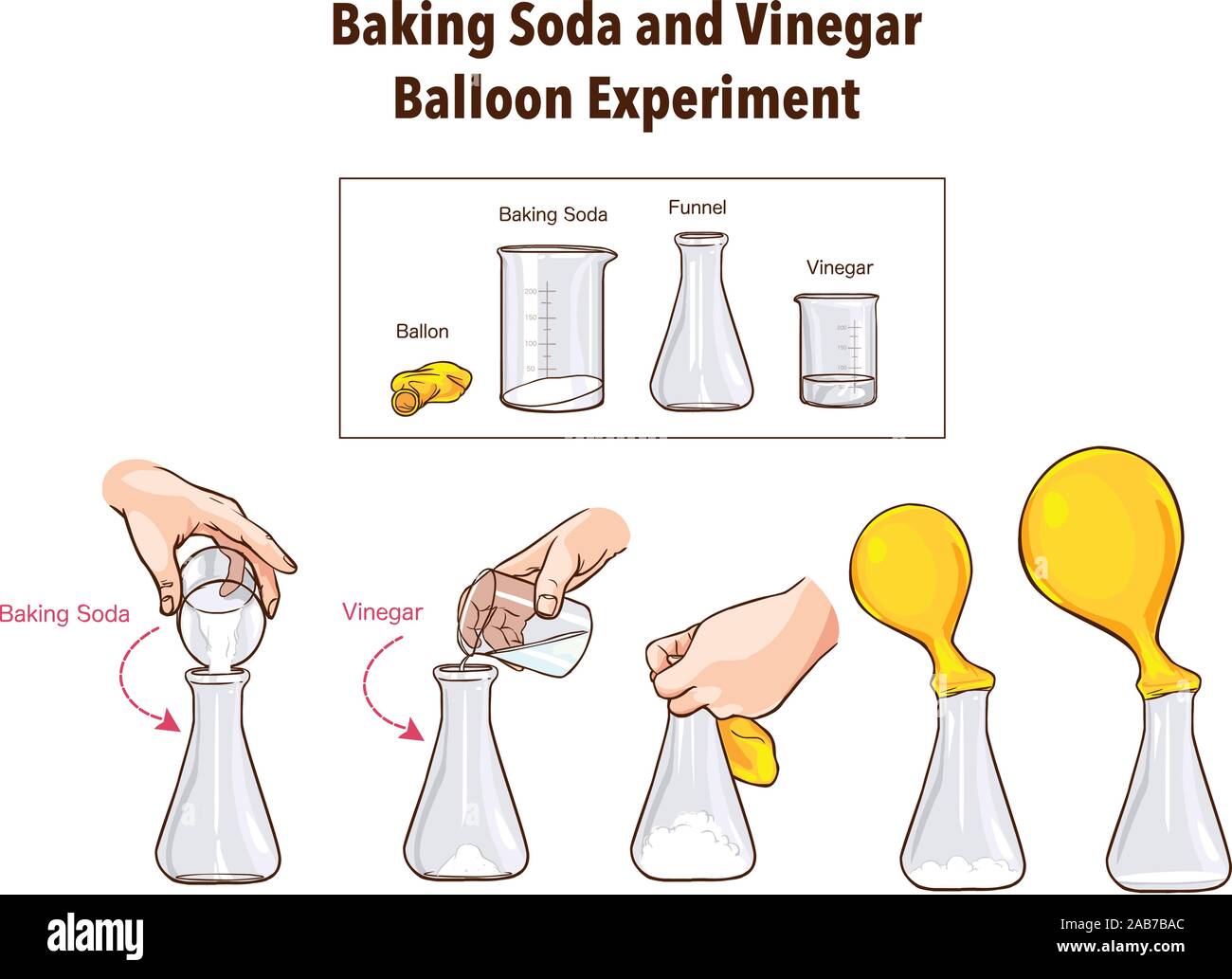
Baking Soda And Vinegar Rate Of Reaction Experiment . This lab demonstrates the reactivity of two household cooking items, baking soda and vinegar. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. The result of this initial reaction is two new chemicals: Carbonic acid and sodium acetate. The second reaction is a decomposition reaction. The remaining atoms create a new. Baking soda is a powdered chemical. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt called sodium acetate. The reaction between vinegar and baking soda is often used in science experiments to demonstrate chemical reactions or to create a gas that can be used to inflate a balloon or.
from www.alamy.com
Baking soda is a powdered chemical. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. The remaining atoms create a new. The reaction between vinegar and baking soda is often used in science experiments to demonstrate chemical reactions or to create a gas that can be used to inflate a balloon or. This lab demonstrates the reactivity of two household cooking items, baking soda and vinegar. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The second reaction is a decomposition reaction. When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt called sodium acetate. Carbonic acid and sodium acetate.
Baking Soda and VinegarBalloon ExperimentScience Stock Vector Image
Baking Soda And Vinegar Rate Of Reaction Experiment The remaining atoms create a new. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. The remaining atoms create a new. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The result of this initial reaction is two new chemicals: Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. The reaction between vinegar and baking soda is often used in science experiments to demonstrate chemical reactions or to create a gas that can be used to inflate a balloon or. Carbonic acid and sodium acetate. Baking soda is a powdered chemical. This lab demonstrates the reactivity of two household cooking items, baking soda and vinegar. The second reaction is a decomposition reaction. When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt called sodium acetate.
From worksheetlistadz.z13.web.core.windows.net
Baking Soda And Vinegar Experiment Worksheet Baking Soda And Vinegar Rate Of Reaction Experiment One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt called sodium acetate. The reaction. Baking Soda And Vinegar Rate Of Reaction Experiment.
From learningschooltrkesp5v.z22.web.core.windows.net
Vinegar Baking Soda Experiment Baking Soda And Vinegar Rate Of Reaction Experiment The result of this initial reaction is two new chemicals: When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.funwithmama.com
Baking Soda And Vinegar Reaction Fun with Mama Baking Soda And Vinegar Rate Of Reaction Experiment One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. The second reaction is a decomposition reaction. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household.. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.alamy.com
Science experiment with baking soda and vinegar balloon illustration Baking Soda And Vinegar Rate Of Reaction Experiment The reaction between vinegar and baking soda is often used in science experiments to demonstrate chemical reactions or to create a gas that can be used to inflate a balloon or. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. When vinegar and baking soda are first mixed. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.vecteezy.com
Baking Soda and Vinegar Balloon Science experiment, Chemistry Baking Soda And Vinegar Rate Of Reaction Experiment This lab demonstrates the reactivity of two household cooking items, baking soda and vinegar. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. Carbonic acid and sodium acetate. Baking soda is a powdered chemical. When vinegar and. Baking Soda And Vinegar Rate Of Reaction Experiment.
From img-abigail.blogspot.com
Experiment Baking Soda And Vinegar Chemical Reaction imgAbigail Baking Soda And Vinegar Rate Of Reaction Experiment One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.youtube.com
How temperature affects the rate of a reaction (vinegar and baking soda Baking Soda And Vinegar Rate Of Reaction Experiment One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. The second reaction is a decomposition reaction. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.eduex.co
rates of reaction EDUEX Baking Soda And Vinegar Rate Of Reaction Experiment The result of this initial reaction is two new chemicals: When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt called sodium acetate. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. The remaining atoms. Baking Soda And Vinegar Rate Of Reaction Experiment.
From fromabcstoacts.com
10 Amazing Baking Soda Science Experiments for Kids From ABCs to ACTs Baking Soda And Vinegar Rate Of Reaction Experiment When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The result of this initial reaction is two new chemicals: The remaining atoms create a new. Carbonic acid and sodium acetate. Baking soda and vinegar react to neutralise each other ( vinegar is an acid. Baking Soda And Vinegar Rate Of Reaction Experiment.
From blogszerotwo.blogspot.com
Baking Soda And Vinegar Experiment All You Need Infos Baking Soda And Vinegar Rate Of Reaction Experiment The result of this initial reaction is two new chemicals: When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The reaction between vinegar and baking soda is often used in science experiments to demonstrate chemical reactions or to create a gas that can be. Baking Soda And Vinegar Rate Of Reaction Experiment.
From img-abigail.blogspot.com
Experiment Baking Soda And Vinegar Chemical Reaction imgAbigail Baking Soda And Vinegar Rate Of Reaction Experiment Baking soda is a powdered chemical. The reaction between vinegar and baking soda is often used in science experiments to demonstrate chemical reactions or to create a gas that can be used to inflate a balloon or. The remaining atoms create a new. The result of this initial reaction is two new chemicals: When you mix baking soda and vinegar. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.scribd.com
Science Rate of Reaction Vinegar + Baking Soda Vinegar Sodium Baking Soda And Vinegar Rate Of Reaction Experiment One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. The result of this initial reaction is two new chemicals: When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium. Baking Soda And Vinegar Rate Of Reaction Experiment.
From logankruwjacobs.blogspot.com
Baking Soda and Vinegar Reaction LogankruwJacobs Baking Soda And Vinegar Rate Of Reaction Experiment One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. Carbonic acid and sodium acetate. When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt. Baking Soda And Vinegar Rate Of Reaction Experiment.
From dbdalrymplesibships.z21.web.core.windows.net
Experiment Vinegar Baking Soda Baking Soda And Vinegar Rate Of Reaction Experiment One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Carbonic acid and. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.youtube.com
Rate Of Reaction Experiment and Explanation Vinegar and Baking Powder Baking Soda And Vinegar Rate Of Reaction Experiment Carbonic acid and sodium acetate. When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt called sodium acetate. This lab demonstrates the reactivity of two household cooking items, baking soda and vinegar. The result of this initial reaction is two new chemicals: The second reaction is a. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.slideshare.net
Baking soda and vinegar experiment report Baking Soda And Vinegar Rate Of Reaction Experiment One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. Carbonic acid and sodium acetate. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.numerade.com
SOLVED Baking soda and vinegar react according to the following Baking Soda And Vinegar Rate Of Reaction Experiment The second reaction is a decomposition reaction. The result of this initial reaction is two new chemicals: One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. In this lab, students will examine the chemical reaction between baking. Baking Soda And Vinegar Rate Of Reaction Experiment.
From craftsbyamanda.com
Colorful Baking Soda and Vinegar Reaction Crafts by Amanda Easy Baking Soda And Vinegar Rate Of Reaction Experiment Carbonic acid and sodium acetate. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. The second reaction is a decomposition reaction. The remaining atoms create a new. When vinegar and baking soda are first mixed together, hydrogen ions. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.alamy.com
Baking Soda and VinegarBalloon ExperimentScience Stock Vector Image Baking Soda And Vinegar Rate Of Reaction Experiment When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt called sodium acetate. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. This lab demonstrates. Baking Soda And Vinegar Rate Of Reaction Experiment.
From ar.inspiredpencil.com
Vinegar And Baking Soda Balloon Experiment Data Baking Soda And Vinegar Rate Of Reaction Experiment The result of this initial reaction is two new chemicals: One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.pinterest.es
The best baking soda and vinegar reaction. Science experiments kids Baking Soda And Vinegar Rate Of Reaction Experiment Baking soda is a powdered chemical. Carbonic acid and sodium acetate. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The result of this initial reaction is two new chemicals: In this lab, students will examine the chemical reaction between baking soda and vinegar,. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.studocu.com
Experiment Class Worksheet Baking Soda and Vinagar Experiment Baking Soda And Vinegar Rate Of Reaction Experiment The second reaction is a decomposition reaction. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Carbonic acid and sodium acetate. The remaining atoms create a new. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions. Baking Soda And Vinegar Rate Of Reaction Experiment.
From preschooleducator.com
ECCE Series Easy Science Experiment Baking Soda and Vinegar Baking Soda And Vinegar Rate Of Reaction Experiment Carbonic acid and sodium acetate. The result of this initial reaction is two new chemicals: One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. When vinegar and baking soda are first mixed together, hydrogen ions in the. Baking Soda And Vinegar Rate Of Reaction Experiment.
From stemtropolis.com
Vinegar and Baking Soda Reaction Experiments STEMtropolis Baking Soda And Vinegar Rate Of Reaction Experiment Baking soda is a powdered chemical. When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt called sodium acetate. Carbonic acid and sodium acetate. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.alamy.com
Baking soda and vinegar experiment Stock Vector Images Alamy Baking Soda And Vinegar Rate Of Reaction Experiment Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. This lab demonstrates the reactivity of two household. Baking Soda And Vinegar Rate Of Reaction Experiment.
From lifeovercs.com
Baking Soda and Vinegar Preschoolers Science Experiment Life Over C's Baking Soda And Vinegar Rate Of Reaction Experiment In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The result of this initial reaction is two new chemicals: The reaction between vinegar. Baking Soda And Vinegar Rate Of Reaction Experiment.
From signalticket9.pythonanywhere.com
Marvelous Vinegar Plus Baking Soda Chemical Equation Balanced For And Baking Soda And Vinegar Rate Of Reaction Experiment The result of this initial reaction is two new chemicals: Carbonic acid and sodium acetate. The remaining atoms create a new. The second reaction is a decomposition reaction. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. The reaction between vinegar and baking soda is often used in. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.youtube.com
Baking Soda & Vinegar Reaction vs. Temperature YouTube Baking Soda And Vinegar Rate Of Reaction Experiment Carbonic acid and sodium acetate. When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar to the baking soda to create a salt called sodium acetate. Baking soda is a powdered chemical. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. This lab. Baking Soda And Vinegar Rate Of Reaction Experiment.
From alfredoyouthweber.blogspot.com
Baking Soda and Vinegar Reaction Baking Soda And Vinegar Rate Of Reaction Experiment The second reaction is a decomposition reaction. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. This lab demonstrates the reactivity of two household cooking items, baking soda and vinegar. The remaining atoms create a new. The reaction between vinegar and baking soda is often used in science. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.youtube.com
Baking soda and vinegar reactionscience experiment Harsh Sirohi Baking Soda And Vinegar Rate Of Reaction Experiment This lab demonstrates the reactivity of two household cooking items, baking soda and vinegar. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. When you mix baking soda and vinegar together, two hydrogen atoms move from the vinegar. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.youtube.com
Type of Reaction for Baking Soda and Vinegar ( NaHCO3 + CH3COOH) YouTube Baking Soda And Vinegar Rate Of Reaction Experiment Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. The result of this initial reaction is two new chemicals: Carbonic acid and sodium acetate. This lab demonstrates the reactivity of two household cooking items, baking soda and vinegar.. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.funwithmama.com
Baking Soda And Vinegar Reaction Fun with Mama Baking Soda And Vinegar Rate Of Reaction Experiment The remaining atoms create a new. The second reaction is a decomposition reaction. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. The result of this initial reaction is two new chemicals: Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.steampoweredfamily.com
10+ Fun and Easy Baking Soda and Vinegar Experiments Baking Soda And Vinegar Rate Of Reaction Experiment When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Baking soda and vinegar react to neutralise each other ( vinegar is an acid. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.vecteezy.com
Baking Soda and Vinegar Balloon Science experiment 21669329 Vector Art Baking Soda And Vinegar Rate Of Reaction Experiment One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Carbonic acid and sodium acetate. Baking soda. Baking Soda And Vinegar Rate Of Reaction Experiment.
From www.science-sparks.com
What is the Baking Soda and Vinegar Reaction? Science Sparks Baking Soda And Vinegar Rate Of Reaction Experiment Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. Baking soda is a powdered chemical. The remaining atoms create a new. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different. Baking Soda And Vinegar Rate Of Reaction Experiment.