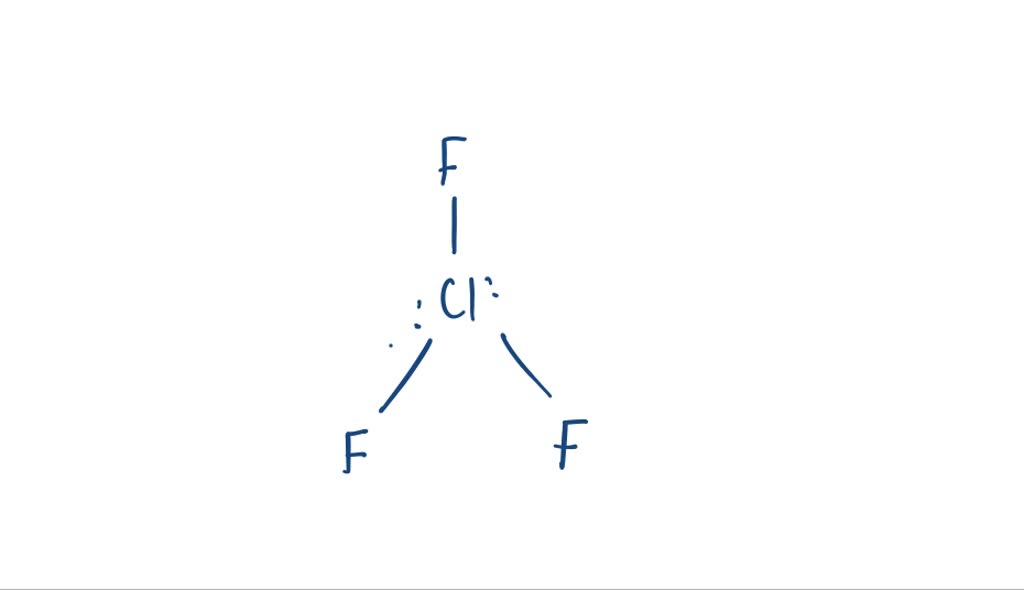
Chlorine Trifluoride Lewis Structure . From the lewis structure, it can be observed that chlorine has an expanded octet. In addition, there are two lone pairs of. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. What is the lewis structure of clf3? There are three fluorine atoms surrounding the central chlorine atom as well. One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. It has two lone pairs attached to it.
from www.numerade.com
Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. In addition, there are two lone pairs of. From the lewis structure, it can be observed that chlorine has an expanded octet. It has two lone pairs attached to it. What is the lewis structure of clf3? The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. There are three fluorine atoms surrounding the central chlorine atom as well. One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure.
SOLVEDChlorine trifluoride, CIF3, is one of the most reactive
Chlorine Trifluoride Lewis Structure Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. It has two lone pairs attached to it. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. In addition, there are two lone pairs of. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. From the lewis structure, it can be observed that chlorine has an expanded octet. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. What is the lewis structure of clf3? There are three fluorine atoms surrounding the central chlorine atom as well.
From www.chegg.com
Solved 5. Molecule CIF, (chlorine trifluoride is an Chlorine Trifluoride Lewis Structure One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. In addition, there are two lone pairs of. It has two lone pairs attached to it. What is the lewis structure of clf3? From the lewis structure,. Chlorine Trifluoride Lewis Structure.
From joipbrcxt.blob.core.windows.net
Chlorine Trifluoride Is Polar Molecule at Sue Hinds blog Chlorine Trifluoride Lewis Structure There are three fluorine atoms surrounding the central chlorine atom as well. What is the lewis structure of clf3? It has two lone pairs attached to it. In addition, there are two lone pairs of. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. One chlorine atom is. Chlorine Trifluoride Lewis Structure.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Trifluoride Lewis Structure From the lewis structure, it can be observed that chlorine has an expanded octet. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. What is the lewis structure of clf3? One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. In addition, there are two lone pairs of.. Chlorine Trifluoride Lewis Structure.
From www.22measured.com
SOLVED The reaction of hydrazine (N2H4) and chlorine trifluoride (ClF3 Chlorine Trifluoride Lewis Structure One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. From the lewis structure, it can be observed that chlorine has an expanded octet. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. There are three fluorine atoms surrounding the central chlorine. Chlorine Trifluoride Lewis Structure.
From www.numerade.com
SOLVED (a) Describe the molecule chlorine dioxide, ClO2, using three Chlorine Trifluoride Lewis Structure What is the lewis structure of clf3? To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. In addition, there are two lone pairs of. From the lewis structure, it can be observed that chlorine has an expanded octet. One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs.. Chlorine Trifluoride Lewis Structure.
From bilag.xxl.no
Draw The Lewis Structure For The Chlorine Trifluoride Molecule Chlorine Trifluoride Lewis Structure The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. It has two lone pairs attached to it. One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine. Chlorine Trifluoride Lewis Structure.
From ck12.org
Molecular Geometry CK12 Foundation Chlorine Trifluoride Lewis Structure From the lewis structure, it can be observed that chlorine has an expanded octet. One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. In addition, there are two lone pairs of. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. There are three fluorine atoms. Chlorine Trifluoride Lewis Structure.
From www.shutterstock.com
Chlorine Trifluoride Molecular Structure Formula Periodic Stock Vector Chlorine Trifluoride Lewis Structure It has two lone pairs attached to it. From the lewis structure, it can be observed that chlorine has an expanded octet. In addition, there are two lone pairs of. There are three fluorine atoms surrounding the central chlorine atom as well. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. The. Chlorine Trifluoride Lewis Structure.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Lewis Structure To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. What is the lewis structure of clf3? The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on. Chlorine Trifluoride Lewis Structure.
From www.fishersci.co.uk
Boron trifluoride dihydrate, 65 BF3, Thermo Scientific Chemicals Chlorine Trifluoride Lewis Structure There are three fluorine atoms surrounding the central chlorine atom as well. In addition, there are two lone pairs of. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. From the lewis structure, it can be observed that chlorine has an expanded octet. It has two lone pairs. Chlorine Trifluoride Lewis Structure.
From www.numerade.com
SOLVED Gaseous chlorine trifluoride is a compound used in the Chlorine Trifluoride Lewis Structure There are three fluorine atoms surrounding the central chlorine atom as well. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. One chlorine atom is covalently bonded to three fluorine. Chlorine Trifluoride Lewis Structure.
From ja-conseil-livres-manga.blogspot.com
Chlorine Pentafluoride Hybridization / The molecule adopts a square Chlorine Trifluoride Lewis Structure There are three fluorine atoms surrounding the central chlorine atom as well. In addition, there are two lone pairs of. It has two lone pairs attached to it. What is the lewis structure of clf3? From the lewis structure, it can be observed that chlorine has an expanded octet. One chlorine atom is covalently bonded to three fluorine atoms, and. Chlorine Trifluoride Lewis Structure.
From slideplayer.com
Molecular Geometry. ppt download Chlorine Trifluoride Lewis Structure Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. What is the lewis structure of clf3? The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine. Chlorine Trifluoride Lewis Structure.
From readingandwritingprojectcom.web.fc2.com
what is the electronic geometry of clf3 Chlorine Trifluoride Lewis Structure One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. What is the. Chlorine Trifluoride Lewis Structure.
From exatin.info
Electron Dot Diagram For Chlorine exatin.info Chlorine Trifluoride Lewis Structure To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. In addition, there are two lone pairs of. What is the lewis structure of clf3? Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine. Chlorine Trifluoride Lewis Structure.
From manualdatasiphonogam.z21.web.core.windows.net
Lewis Diagram Of Chlorine Chlorine Trifluoride Lewis Structure Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. What is the lewis structure of clf3? In addition, there are two lone pairs of. There are three fluorine atoms surrounding the central chlorine atom as well.. Chlorine Trifluoride Lewis Structure.
From www.studocu.com
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu Chlorine Trifluoride Lewis Structure What is the lewis structure of clf3? In addition, there are two lone pairs of. There are three fluorine atoms surrounding the central chlorine atom as well. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. The. Chlorine Trifluoride Lewis Structure.
From thvinhtuy.edu.vn
Lewis structure Chlorine Valence electron Diagram, 18, chemical Element Chlorine Trifluoride Lewis Structure It has two lone pairs attached to it. There are three fluorine atoms surrounding the central chlorine atom as well. What is the lewis structure of clf3? In addition, there are two lone pairs of. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. One chlorine atom is covalently bonded to three fluorine atoms,. Chlorine Trifluoride Lewis Structure.
From www.studocu.com
Chemistry 101 Chapter 7 Part 3 Draw the Lewis structure of ClF₃ Chlorine Trifluoride Lewis Structure In addition, there are two lone pairs of. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. There are three fluorine atoms surrounding the central chlorine atom as well. It has two lone pairs attached to it. One chlorine atom is covalently bonded to three fluorine atoms, and. Chlorine Trifluoride Lewis Structure.
From alchetron.com
Chlorine trifluoride Alchetron, The Free Social Encyclopedia Chlorine Trifluoride Lewis Structure From the lewis structure, it can be observed that chlorine has an expanded octet. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. What is the lewis structure of clf3?. Chlorine Trifluoride Lewis Structure.
From www.numerade.com
SOLVEDChlorine trifluoride, CIF3, is one of the most reactive Chlorine Trifluoride Lewis Structure It has two lone pairs attached to it. What is the lewis structure of clf3? The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms.. Chlorine Trifluoride Lewis Structure.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Lewis Structure The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. From the lewis structure, it can be observed that chlorine has an expanded octet. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. One chlorine atom is covalently. Chlorine Trifluoride Lewis Structure.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Trifluoride Lewis Structure Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. What is the lewis structure of clf3? In addition, there are two lone pairs of. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. There are three fluorine atoms surrounding the central chlorine atom as well. It. Chlorine Trifluoride Lewis Structure.
From ajorpng.blogspot.com
Chlorine Pentafluoride Lewis / Chlorine pentafluoride is a square Chlorine Trifluoride Lewis Structure Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. There are three fluorine. Chlorine Trifluoride Lewis Structure.
From www.youtube.com
ClF3 Lewis Structure (Chlorine Trifluoride) YouTube Chlorine Trifluoride Lewis Structure It has two lone pairs attached to it. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis. Chlorine Trifluoride Lewis Structure.
From cartoondealer.com
Chlorine Molecule RoyaltyFree Stock Photo 3900921 Chlorine Trifluoride Lewis Structure What is the lewis structure of clf3? Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. It has two lone pairs attached to it. In addition, there are two lone pairs of. One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. To determine the molecular. Chlorine Trifluoride Lewis Structure.
From www.kindpng.com
Chlorine Trifluoride 3d Balls Chlorine Trifluoride 3d Structure, HD Chlorine Trifluoride Lewis Structure What is the lewis structure of clf3? It has two lone pairs attached to it. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. From the lewis structure, it can be observed that. Chlorine Trifluoride Lewis Structure.
From www.slideserve.com
PPT VSEPR. PowerPoint Presentation ID214953 Chlorine Trifluoride Lewis Structure To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. From the lewis structure, it. Chlorine Trifluoride Lewis Structure.
From imgbin.com
Lewis Structure Electron Chlorine Diagram Chloride PNG, Clipart, Black Chlorine Trifluoride Lewis Structure There are three fluorine atoms surrounding the central chlorine atom as well. The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. In addition, there are two lone pairs of. To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. One. Chlorine Trifluoride Lewis Structure.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Trifluoride Lewis Structure The lewis structure for clf3 (chlorine trifluoride) involves three bonds between the chlorine and fluorine atoms, with two lone pairs on the chlorine atom. What is the lewis structure of clf3? To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. Hi guys!welcome to our channel in today's video we are going to help you. Chlorine Trifluoride Lewis Structure.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Lewis Structure Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. What is the lewis structure of clf3? There are three fluorine atoms surrounding the central chlorine atom as well. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. It has two. Chlorine Trifluoride Lewis Structure.
From imgbin.com
Interhalogen Bromine Pentafluoride Chlorine Trifluoride Iodine Chlorine Trifluoride Lewis Structure From the lewis structure, it can be observed that chlorine has an expanded octet. In addition, there are two lone pairs of. One chlorine atom is covalently bonded to three fluorine atoms, and also has two lone pairs. It has two lone pairs attached to it. Hi guys!welcome to our channel in today's video we are going to help you. Chlorine Trifluoride Lewis Structure.
From www.chegg.com
Solved Draw Lewis structures for each molecule. chlorine Chlorine Trifluoride Lewis Structure To determine the molecular geometry for chlorine trifluoride, we go back to its lewis structure. From the lewis structure, it can be observed that chlorine has an expanded octet. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. The lewis structure for clf3 (chlorine trifluoride) involves three bonds. Chlorine Trifluoride Lewis Structure.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Trifluoride Lewis Structure In addition, there are two lone pairs of. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. From the lewis structure, it can be observed that chlorine has an expanded octet. It has two lone pairs attached to it. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom. Chlorine Trifluoride Lewis Structure.
From www.shutterstock.com
Lewis Structure Chlorine Cl Stock Vector (Royalty Free) 2115881024 Chlorine Trifluoride Lewis Structure The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Hi guys!welcome to our channel in today's video we are going to help you determine the lewis. There are three fluorine atoms surrounding the central chlorine atom as well. One chlorine atom is covalently bonded to three fluorine atoms,. Chlorine Trifluoride Lewis Structure.