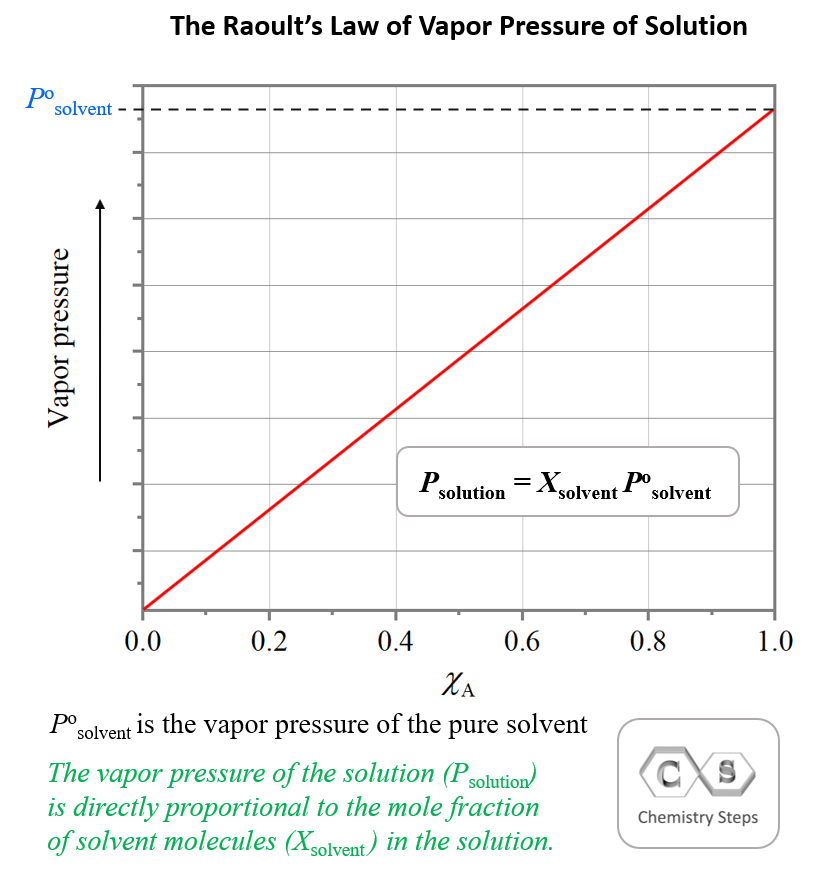
Suspension Lower Vapor Pressure . (the exceptions to this statement are if the solute itself is a liquid or. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The vapor pressure lowering is directly. The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. The chemical nature of the solute is not important because the vapor pressure is. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. We can solve vapor pressure problems in either of two ways: That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. Relative lowering of vapour pressure.
from general.chemistrysteps.com
The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The vapor pressure lowering is directly. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. We can solve vapor pressure problems in either of two ways: The chemical nature of the solute is not important because the vapor pressure is. Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);
Vapor Pressure Lowering Chemistry Steps
Suspension Lower Vapor Pressure The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. Relative lowering of vapour pressure. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The chemical nature of the solute is not important because the vapor pressure is. The vapor pressure lowering is directly. We can solve vapor pressure problems in either of two ways: (the exceptions to this statement are if the solute itself is a liquid or. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly.
From www.slideserve.com
PPT AP Chemistry Notes PowerPoint Presentation, free download ID Suspension Lower Vapor Pressure Relative lowering of vapour pressure. The vapor pressure lowering is directly. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The chemical nature of the solute is not important because the vapor pressure. Suspension Lower Vapor Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Suspension Lower Vapor Pressure The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. Relative lowering of vapour pressure. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample.. Suspension Lower Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Suspension Lower Vapor Pressure The vapor pressure lowering is directly. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates. Suspension Lower Vapor Pressure.
From www.youtube.com
VaporPressure Lowering (Raoult's Law) ΔP = XBP°A YouTube Suspension Lower Vapor Pressure That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The presence of a dissolved solid lowers the characteristic vapor pressure of a. Suspension Lower Vapor Pressure.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Suspension Lower Vapor Pressure The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The vapor. Suspension Lower Vapor Pressure.
From www.youtube.com
Colligative properties Vapor pressure lowering YouTube Suspension Lower Vapor Pressure The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The chemical nature of the solute is not important because the vapor pressure is. The vapor pressure lowering is directly. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of a liquid is the equilibrium pressure of. Suspension Lower Vapor Pressure.
From jmpcoblog.com
Avoiding Pump Cavitation in Open Systems The Dynamic Relationship Suspension Lower Vapor Pressure Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The vapor. Suspension Lower Vapor Pressure.
From www.chegg.com
Solved The higher the relative humidity and air temperature, Suspension Lower Vapor Pressure The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. (the exceptions to this statement are if the solute itself is a liquid or. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT SOLUTIONS PowerPoint Presentation, free download ID1598971 Suspension Lower Vapor Pressure We can solve vapor pressure problems in either of two ways: By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The chemical nature of the solute is not important because the vapor pressure is. (the exceptions to. Suspension Lower Vapor Pressure.
From saylordotorg.github.io
Vapor Pressure Suspension Lower Vapor Pressure (the exceptions to this statement are if the solute itself is a liquid or. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. Vapor pressure is defined as the partial pressure of a vapor in. Suspension Lower Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Suspension Lower Vapor Pressure The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure lowering is directly. The chemical nature of the solute is not important because the vapor pressure is. We can solve vapor pressure problems in either. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT Vapor Pressure Lowering PowerPoint Presentation, free download Suspension Lower Vapor Pressure By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. We can solve vapor pressure problems in either of two ways: Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid. Suspension Lower Vapor Pressure.
From www.ck12.org
VaporPressure Lowering (Raoult's Law) ΔP = X[B]P°[A] Example 1 Suspension Lower Vapor Pressure The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The lowering of the vapor pressure depends on the number of. Suspension Lower Vapor Pressure.
From scienceinfo.com
Lowering of Vapor Pressure A Colligative property Suspension Lower Vapor Pressure Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download Suspension Lower Vapor Pressure The vapor pressure lowering is directly. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); By. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download Suspension Lower Vapor Pressure The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2965441 Suspension Lower Vapor Pressure Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. The vapor pressure of a liquid is the equilibrium pressure of. Suspension Lower Vapor Pressure.
From chemistnotes.com
Vapor Pressure, Lowering of Vapor Pressure, Definition, Equation Suspension Lower Vapor Pressure (the exceptions to this statement are if the solute itself is a liquid or. We can solve vapor pressure problems in either of two ways: Relative lowering of vapour pressure. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The chemical nature of the solute is not important because the vapor pressure. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3588530 Suspension Lower Vapor Pressure The vapor pressure lowering is directly. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The vapor pressure of a solvent in a solution is always lower than. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download Suspension Lower Vapor Pressure (the exceptions to this statement are if the solute itself is a liquid or. We can solve vapor pressure problems in either of two ways: By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The vapor pressure lowering is directly.. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT Molality and Mole Fraction PowerPoint Presentation ID259070 Suspension Lower Vapor Pressure By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. We can solve vapor pressure problems in either of two ways: That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure lowering is directly. The chemical nature of the solute is not important because the vapor pressure. Suspension Lower Vapor Pressure.
From www.youtube.com
What is vapor pressure lowering? YouTube Suspension Lower Vapor Pressure That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The vapor pressure lowering is directly. (the exceptions to this statement are if the solute itself is a liquid or. Relative lowering of vapour pressure.. Suspension Lower Vapor Pressure.
From inspiritvr.com
Vapor Pressure Lowering Study Guide Inspirit Suspension Lower Vapor Pressure The chemical nature of the solute is not important because the vapor pressure is. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. The vapor pressure lowering is directly. That is,. Suspension Lower Vapor Pressure.
From slideplayer.com
Chemistry ppt download Suspension Lower Vapor Pressure Relative lowering of vapour pressure. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. (the exceptions to this statement are if the solute itself is a liquid or. The vapor pressure lowering. Suspension Lower Vapor Pressure.
From nigerianscholars.com
Vapor Pressure Lowering Solutions and Colloids Suspension Lower Vapor Pressure We can solve vapor pressure problems in either of two ways: The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. (the exceptions to this statement are if the solute itself is a liquid or. The lowering of the vapor pressure depends on the number of solute particles that have. Suspension Lower Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Suspension Lower Vapor Pressure The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. (the exceptions to this statement. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 Suspension Lower Vapor Pressure The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid or liquid) at a. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT III. Colligative Properties PowerPoint Presentation, free Suspension Lower Vapor Pressure (the exceptions to this statement are if the solute itself is a liquid or. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. The lowering of the vapor pressure depends on. Suspension Lower Vapor Pressure.
From www.shutterstock.com
Colligative Properties Vapor Pressure Lowering Solution vetor stock Suspension Lower Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Relative lowering of vapour pressure. (the exceptions to this statement are if the solute itself is a liquid or. The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. Vapor pressure is. Suspension Lower Vapor Pressure.
From slideplayer.com
Chapter 16 Mixtures & Solutions ppt download Suspension Lower Vapor Pressure Relative lowering of vapour pressure. We can solve vapor pressure problems in either of two ways: (the exceptions to this statement are if the solute itself is a liquid or. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The lowering of the vapor pressure depends on the number. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2965441 Suspension Lower Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. Vapor pressure is defined as the partial pressure of a vapor in dynamic equilibrium with its condensed phases (solid or liquid) at a given temperature. Suspension Lower Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Suspension Lower Vapor Pressure The vapor pressure lowering is directly. The chemical nature of the solute is not important because the vapor pressure is. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. The lowering of the vapor. Suspension Lower Vapor Pressure.
From www.slideserve.com
PPT Properties of Solutions PowerPoint Presentation, free download Suspension Lower Vapor Pressure By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. Vapor pressure is defined as the partial pressure of a vapor in dynamic. Suspension Lower Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Suspension Lower Vapor Pressure The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Relative lowering. Suspension Lower Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Suspension Lower Vapor Pressure The lowering of the vapor pressure depends on the number of solute particles that have been dissolved. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The lowering of the vapor pressure depends on the number of solute particles. Suspension Lower Vapor Pressure.