What Is The Experimental Boiling Point Of Water . If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Under this condition, addition of. You could heat each of the liquids and measure their boiling points with. If you add salt to water, you raise the boiling point, or the temperature at which water boils. In this experiment you will test the effect of table salt (sodium chloride) on the boiling point of water. Updated on july 13, 2024. Whether you want to analyze. Impurities in water, like salt, modify the intermolecular interactions between water molecules that result in a modified boiling point. Pure water boils at 100°c at normal atmospheric pressure. You may repeat this experiment. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; As solute molecules are added to water, the boiling point increases.
from www.youtube.com
The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. Whether you want to analyze. You may repeat this experiment. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; Updated on july 13, 2024. You could heat each of the liquids and measure their boiling points with. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. As solute molecules are added to water, the boiling point increases.
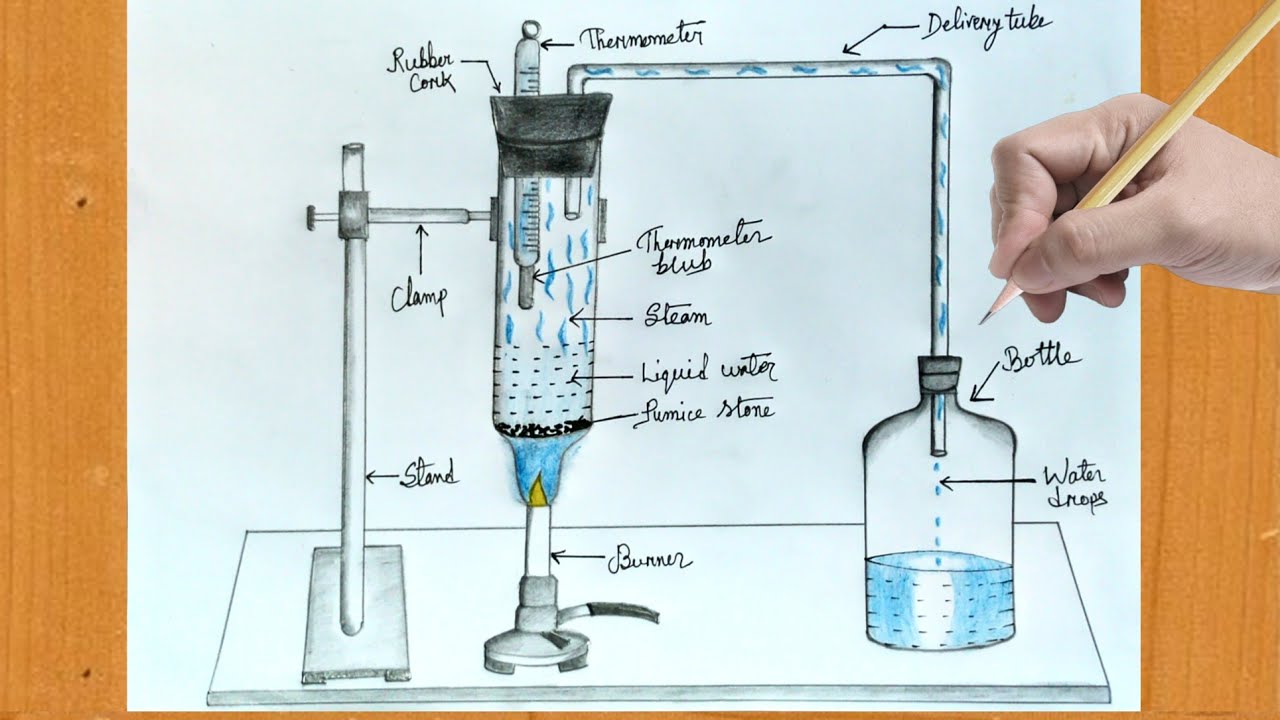
How To Draw Determination Of Boiling Point Of Water Diagram Easily Step
What Is The Experimental Boiling Point Of Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. Pure water boils at 100°c at normal atmospheric pressure. As solute molecules are added to water, the boiling point increases. Updated on july 13, 2024. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If you add salt to water, you raise the boiling point, or the temperature at which water boils. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. In this experiment you will test the effect of table salt (sodium chloride) on the boiling point of water. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; You could heat each of the liquids and measure their boiling points with. Whether you want to analyze. You may repeat this experiment. Impurities in water, like salt, modify the intermolecular interactions between water molecules that result in a modified boiling point. Under this condition, addition of.
From www.youtube.com
Does Salt Water Boil Faster? Experiment YouTube What Is The Experimental Boiling Point Of Water You may repeat this experiment. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Pure water boils at 100°c at normal atmospheric pressure. Whether you want to analyze. Under this condition, addition of. Updated on july 13, 2024. As solute molecules are added to water, the boiling. What Is The Experimental Boiling Point Of Water.
From montoguequiz.com
Boiling and Condensation Solved Problems What Is The Experimental Boiling Point Of Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid. What Is The Experimental Boiling Point Of Water.
From www.youtube.com
Boiling point determination Exp 1 YouTube What Is The Experimental Boiling Point Of Water Under this condition, addition of. Whether you want to analyze. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; In this experiment you will test the effect of table salt (sodium chloride) on the boiling point of water. Updated on july 13, 2024.. What Is The Experimental Boiling Point Of Water.
From www.youtube.com
Boiling Point Experiment S21 YouTube What Is The Experimental Boiling Point Of Water Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; You may repeat this experiment. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will increase by about 0.5 c. What Is The Experimental Boiling Point Of Water.
From www.youtube.com
Boiling Point Determination YouTube What Is The Experimental Boiling Point Of Water As solute molecules are added to water, the boiling point increases. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Whether you want to analyze. Pure water boils at 100°c at normal atmospheric pressure. You may repeat this experiment. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling. What Is The Experimental Boiling Point Of Water.
From www.shutterstock.com
Temperature Ice And Boiling Water Stock Vector 303198758 Shutterstock What Is The Experimental Boiling Point Of Water Updated on july 13, 2024. As solute molecules are added to water, the boiling point increases. Pure water boils at 100°c at normal atmospheric pressure. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. If you add salt to water, you. What Is The Experimental Boiling Point Of Water.
From mungfali.com
Water Boiling Point Pressure Chart What Is The Experimental Boiling Point Of Water Updated on july 13, 2024. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. Impurities in water, like salt, modify the intermolecular interactions between water molecules that result in a modified boiling point. You may repeat this experiment. The boiling point. What Is The Experimental Boiling Point Of Water.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is The Experimental Boiling Point Of Water Pure water boils at 100°c at normal atmospheric pressure. You may repeat this experiment. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. Updated on july 13, 2024. Under this condition, addition of. Impurities in water, like salt, modify the intermolecular. What Is The Experimental Boiling Point Of Water.
From heatingwaterdzumekuchi.blogspot.com
Heating Water Heating Water Experiment What Is The Experimental Boiling Point Of Water In this experiment you will test the effect of table salt (sodium chloride) on the boiling point of water. Impurities in water, like salt, modify the intermolecular interactions between water molecules that result in a modified boiling point. As solute molecules are added to water, the boiling point increases. The temperature needed to boil will increase by about 0.5 c. What Is The Experimental Boiling Point Of Water.
From www.youtube.com
Calculating boiling point elevation YouTube What Is The Experimental Boiling Point Of Water You may repeat this experiment. Whether you want to analyze. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Impurities in water, like salt, modify the intermolecular interactions between water molecules that result in a modified boiling point. If you want a quick and simple answer, you. What Is The Experimental Boiling Point Of Water.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is The Experimental Boiling Point Of Water The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Pure water boils at 100°c at normal atmospheric pressure. You could heat each of the liquids and measure their boiling. What Is The Experimental Boiling Point Of Water.
From labonlaptop.com
Find Boiling Point of Water labOnLaptop Store What Is The Experimental Boiling Point Of Water Under this condition, addition of. As solute molecules are added to water, the boiling point increases. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. You may repeat this experiment. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by. What Is The Experimental Boiling Point Of Water.
From worksheetzonebellini.z5.web.core.windows.net
Salt And Boiling Water Experiment What Is The Experimental Boiling Point Of Water You may repeat this experiment. Whether you want to analyze. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at. What Is The Experimental Boiling Point Of Water.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is The Experimental Boiling Point Of Water You could heat each of the liquids and measure their boiling points with. Whether you want to analyze. Impurities in water, like salt, modify the intermolecular interactions between water molecules that result in a modified boiling point. If you add salt to water, you raise the boiling point, or the temperature at which water boils. If you want a quick. What Is The Experimental Boiling Point Of Water.
From ar.inspiredpencil.com
Boiling Point Of Water Examples What Is The Experimental Boiling Point Of Water You may repeat this experiment. You could heat each of the liquids and measure their boiling points with. Updated on july 13, 2024. In this experiment you will test the effect of table salt (sodium chloride) on the boiling point of water. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the. What Is The Experimental Boiling Point Of Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Experimental Boiling Point Of Water Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. You could heat each of the. What Is The Experimental Boiling Point Of Water.
From www.youtube.com
How To Draw Determination Of Boiling Point Of Water Diagram Easily Step What Is The Experimental Boiling Point Of Water Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; Updated on july 13, 2024. As solute molecules are added to water, the boiling point increases. If you add salt to water, you raise the boiling point, or the temperature at which water boils.. What Is The Experimental Boiling Point Of Water.
From madalyn-has-dorsey.blogspot.com
Boiling Point of Water MadalynhasDorsey What Is The Experimental Boiling Point Of Water In this experiment you will test the effect of table salt (sodium chloride) on the boiling point of water. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. You may repeat this experiment. If you add salt to water, you raise. What Is The Experimental Boiling Point Of Water.
From www.youtube.com
boiling point experiment YouTube What Is The Experimental Boiling Point Of Water The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Pure water boils at 100°c at normal atmospheric pressure. As solute molecules are added to water, the boiling point increases. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Impurities in. What Is The Experimental Boiling Point Of Water.
From byjus.com
Determination Of Boiling Point Of An Organic Compound Chemistry What Is The Experimental Boiling Point Of Water As solute molecules are added to water, the boiling point increases. Impurities in water, like salt, modify the intermolecular interactions between water molecules that result in a modified boiling point. Under this condition, addition of. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1. What Is The Experimental Boiling Point Of Water.
From www.animalia-life.club
Boiling Point Of Water For Kids What Is The Experimental Boiling Point Of Water Updated on july 13, 2024. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. The boiling point for water is 212 ºf or 100 ºc,. What Is The Experimental Boiling Point Of Water.
From www.researchgate.net
(a) Schematic phase diagram of ethanol. TP, BP, and CP are the triple What Is The Experimental Boiling Point Of Water As solute molecules are added to water, the boiling point increases. In this experiment you will test the effect of table salt (sodium chloride) on the boiling point of water. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Pure water boils at 100°c at normal atmospheric pressure. Whether you want to analyze.. What Is The Experimental Boiling Point Of Water.
From www.youtube.com
Chemistry Animation Salt in Boiling Water.wmv YouTube What Is The Experimental Boiling Point Of Water Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; Whether you want to analyze. In this experiment you will test the effect of table salt (sodium chloride) on the boiling point of water. Under this condition, addition of. If you add salt to. What Is The Experimental Boiling Point Of Water.
From www.youtube.com
Lab 18.2 Adding Salt to Boiling Water YouTube What Is The Experimental Boiling Point Of Water As solute molecules are added to water, the boiling point increases. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. If you add salt to water, you raise the boiling point, or the temperature at which water boils. You could heat each of the liquids and measure. What Is The Experimental Boiling Point Of Water.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and What Is The Experimental Boiling Point Of Water In this experiment you will test the effect of table salt (sodium chloride) on the boiling point of water. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Impurities in water, like salt, modify the intermolecular interactions between water molecules that result in a modified boiling point.. What Is The Experimental Boiling Point Of Water.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of What Is The Experimental Boiling Point Of Water Impurities in water, like salt, modify the intermolecular interactions between water molecules that result in a modified boiling point. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. You may repeat this experiment. In this experiment you will test the effect. What Is The Experimental Boiling Point Of Water.
From jyoukhanatask1.weebly.com
Investigating the Boiling Point of water What Is The Experimental Boiling Point Of Water You may repeat this experiment. In this experiment you will test the effect of table salt (sodium chloride) on the boiling point of water. Whether you want to analyze. You could heat each of the liquids and measure their boiling points with. Updated on july 13, 2024. Under this condition, addition of. Pure water boils at 100°c at normal atmospheric. What Is The Experimental Boiling Point Of Water.
From www.thoughtco.com
What Is the Boiling Point of Water? What Is The Experimental Boiling Point Of Water Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; You could heat each of the liquids and measure their boiling points with. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed. What Is The Experimental Boiling Point Of Water.
From www.chegg.com
Solved The boiling point of water H2O is 100.0∘C at 1 What Is The Experimental Boiling Point Of Water You may repeat this experiment. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is. What Is The Experimental Boiling Point Of Water.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Is The Experimental Boiling Point Of Water Under this condition, addition of. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure. You could heat each of the liquids and measure their boiling points with. Impurities in water, like salt, modify the intermolecular interactions between water molecules that result. What Is The Experimental Boiling Point Of Water.
From mavink.com
Water Boiling Point Pressure Chart What Is The Experimental Boiling Point Of Water Whether you want to analyze. You may repeat this experiment. You could heat each of the liquids and measure their boiling points with. As solute molecules are added to water, the boiling point increases. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Under this condition, addition. What Is The Experimental Boiling Point Of Water.
From www.dreamstime.com
Science Experiment with Boiling Water on the Rack Stock Vector What Is The Experimental Boiling Point Of Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. As solute molecules are added to water, the boiling point increases. Impurities in water, like salt, modify the intermolecular interactions between water molecules that result in a modified boiling point. The temperature needed to boil will increase by about 0.5 c for. What Is The Experimental Boiling Point Of Water.
From scienceblogs.com
Water in Space What Happens? ScienceBlogs What Is The Experimental Boiling Point Of Water The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Whether you want to analyze. Updated on july 13, 2024. If you want a quick and simple answer, you can say that the boiling point of. What Is The Experimental Boiling Point Of Water.
From spikaph.blogspot.com
Boiling Point Of Water Boiling point of water YouTube For What Is The Experimental Boiling Point Of Water You may repeat this experiment. Updated on july 13, 2024. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Under this condition, addition of. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f. What Is The Experimental Boiling Point Of Water.
From www.vernier.com
Boiling Temperature of Water > Experiment 3 from Exploring Physical Science What Is The Experimental Boiling Point Of Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. Updated on july 13, 2024. You may repeat this experiment. Whether you want to analyze. If you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of. What Is The Experimental Boiling Point Of Water.