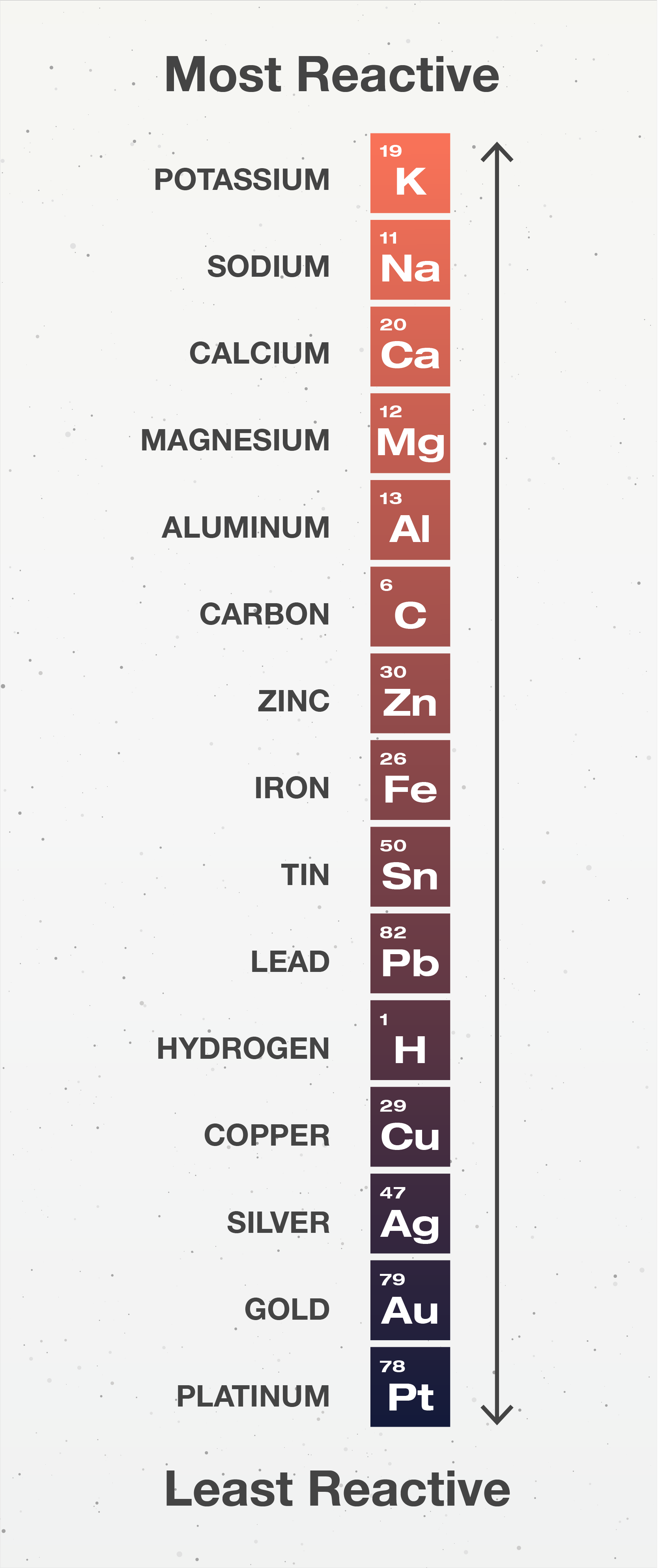
Which Metal Is The Most Active . The greater the shielding, the greater the ability to lose electrons. The more easily it loses electrons to form positive. Which alkali metal should be the most active? The more vigorously it reacts with other substances. Should this element have the most or least negative value for its standard reduction potential? In general, the bigger the atom, the greater the ability to lose electrons. They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. For any two metals in the series, the metal placed higher in the series. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. These are the most reactive metals and readily lose electrons to form cations. Therefore, metallic character increases going down the table,. 9 rows in general, the more reactive a metal is: Most reactive metals are at the top while the least reactive metals at the bottom. The most reactive metal on the periodic table is francium.
from www.freeexamacademy.com
They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. Should this element have the most or least negative value for its standard reduction potential? These are the most reactive metals and readily lose electrons to form cations. 9 rows in general, the more reactive a metal is: The more vigorously it reacts with other substances. The more easily it loses electrons to form positive. For any two metals in the series, the metal placed higher in the series. The greater the shielding, the greater the ability to lose electrons. The most reactive metal on the periodic table is francium.
Metals Free Exam Academy
Which Metal Is The Most Active In general, the bigger the atom, the greater the ability to lose electrons. Most reactive metals are at the top while the least reactive metals at the bottom. Should this element have the most or least negative value for its standard reduction potential? 9 rows in general, the more reactive a metal is: The more vigorously it reacts with other substances. In general, the bigger the atom, the greater the ability to lose electrons. These are the most reactive metals and readily lose electrons to form cations. Therefore, metallic character increases going down the table,. Which alkali metal should be the most active? They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. For any two metals in the series, the metal placed higher in the series. The more easily it loses electrons to form positive. The most reactive metal on the periodic table is francium. The greater the shielding, the greater the ability to lose electrons.
From www.slideserve.com
PPT The Periodic Properties of the Elements PowerPoint Presentation Which Metal Is The Most Active The more vigorously it reacts with other substances. These are the most reactive metals and readily lose electrons to form cations. For any two metals in the series, the metal placed higher in the series. Therefore, metallic character increases going down the table,. Which alkali metal should be the most active? The most active metals are so reactive that they. Which Metal Is The Most Active.
From elchoroukhost.net
List Of All Metal Elements In The Periodic Table Elcho Table Which Metal Is The Most Active For any two metals in the series, the metal placed higher in the series. Therefore, metallic character increases going down the table,. These are the most reactive metals and readily lose electrons to form cations. The more easily it loses electrons to form positive. 9 rows in general, the more reactive a metal is: The greater the shielding, the greater. Which Metal Is The Most Active.
From www.nagwa.com
Question Video Determining Which Metal Is the Most Active Nagwa Which Metal Is The Most Active Therefore, metallic character increases going down the table,. The more easily it loses electrons to form positive. They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. 9 rows in general, the more reactive a metal is: The more vigorously it reacts with other substances. These are the most reactive metals and readily lose. Which Metal Is The Most Active.
From periodictable.me
Most reactive metal is… Which Metal Is The Most Active In general, the bigger the atom, the greater the ability to lose electrons. The most reactive metal on the periodic table is francium. The greater the shielding, the greater the ability to lose electrons. The more vigorously it reacts with other substances. They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. Therefore, metallic. Which Metal Is The Most Active.
From www.slideserve.com
PPT Acid Base Reactions PowerPoint Presentation, free download ID Which Metal Is The Most Active In general, the bigger the atom, the greater the ability to lose electrons. For any two metals in the series, the metal placed higher in the series. Most reactive metals are at the top while the least reactive metals at the bottom. Which alkali metal should be the most active? Should this element have the most or least negative value. Which Metal Is The Most Active.
From garetpay.weebly.com
Transition metals reactivity garetpay Which Metal Is The Most Active The most reactive metal on the periodic table is francium. Most reactive metals are at the top while the least reactive metals at the bottom. The more easily it loses electrons to form positive. These are the most reactive metals and readily lose electrons to form cations. 9 rows in general, the more reactive a metal is: Therefore, metallic character. Which Metal Is The Most Active.
From www.pinterest.com
The Metal Reactivity Series Chemistry classroom Which Metal Is The Most Active Therefore, metallic character increases going down the table,. They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. Which alkali metal should be the most active? In general, the bigger the atom, the greater the ability to lose electrons. The most active metals are so reactive that they readily combine with the o 2. Which Metal Is The Most Active.
From brokeasshome.com
Where Are The Most Active Metals Located Periodic Table Worksheet Which Metal Is The Most Active 9 rows in general, the more reactive a metal is: In general, the bigger the atom, the greater the ability to lose electrons. Should this element have the most or least negative value for its standard reduction potential? Therefore, metallic character increases going down the table,. These are the most reactive metals and readily lose electrons to form cations. Which. Which Metal Is The Most Active.
From precious-well-baker.blogspot.com
The Most Active Metals Are Located in the Which Metal Is The Most Active 9 rows in general, the more reactive a metal is: These are the most reactive metals and readily lose electrons to form cations. The more vigorously it reacts with other substances. Most reactive metals are at the top while the least reactive metals at the bottom. The most reactive metal on the periodic table is francium. The more easily it. Which Metal Is The Most Active.
From www.slideserve.com
PPT Predicting Products The Activity Series & Solubility Rules Which Metal Is The Most Active The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. Which alkali metal should be the most active? These are the most reactive metals and readily lose electrons to form cations. They are found in groups ia (alkali metals) and iia. Which Metal Is The Most Active.
From brainly.in
) Arrange these metals in decreasing order of their reactivity Zn, Al Which Metal Is The Most Active These are the most reactive metals and readily lose electrons to form cations. 9 rows in general, the more reactive a metal is: They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. The more easily it loses electrons to form positive. The most reactive metal on the periodic table is francium. The most. Which Metal Is The Most Active.
From www.chemistrystudent.com
Group 2 Metal Reactions (ALevel) ChemistryStudent Which Metal Is The Most Active For any two metals in the series, the metal placed higher in the series. The more easily it loses electrons to form positive. The more vigorously it reacts with other substances. Which alkali metal should be the most active? In general, the bigger the atom, the greater the ability to lose electrons. The most reactive metal on the periodic table. Which Metal Is The Most Active.
From www.walmart.com
Active Mine Keep Out Weatherproof Metal Sign SIZE 8" x 12" Which Metal Is The Most Active The most reactive metal on the periodic table is francium. The more vigorously it reacts with other substances. Therefore, metallic character increases going down the table,. For any two metals in the series, the metal placed higher in the series. The more easily it loses electrons to form positive. Most reactive metals are at the top while the least reactive. Which Metal Is The Most Active.
From sciencenotes.org
Activity Series of Metals (Reactivity Series) Which Metal Is The Most Active Which alkali metal should be the most active? They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. The more easily it loses electrons to form positive. The more vigorously it reacts with other substances. These are the most reactive metals and readily lose electrons to form cations. For any two metals in the. Which Metal Is The Most Active.
From brokeasshome.com
Periodic Table Worksheet Answers Where Are The Most Active Metals Which Metal Is The Most Active The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. The more easily it loses electrons to form positive. These are the most reactive metals and readily lose electrons to form cations. For any two metals in the series, the metal. Which Metal Is The Most Active.
From mavink.com
Periodic Table Most Reactive Metals Which Metal Is The Most Active In general, the bigger the atom, the greater the ability to lose electrons. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. The more easily it loses electrons to form positive. Most reactive metals are at the top while the. Which Metal Is The Most Active.
From www.adda247.com
Reactivity series of Metals and Non Metals Which Metal Is The Most Active The most reactive metal on the periodic table is francium. The greater the shielding, the greater the ability to lose electrons. Should this element have the most or least negative value for its standard reduction potential? Most reactive metals are at the top while the least reactive metals at the bottom. The more easily it loses electrons to form positive.. Which Metal Is The Most Active.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table Which Metal Is The Most Active In general, the bigger the atom, the greater the ability to lose electrons. Most reactive metals are at the top while the least reactive metals at the bottom. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. The greater the. Which Metal Is The Most Active.
From askfilo.com
Wi Name the most and least active metal.(ii) Arrange the metals A,B,C and.. Which Metal Is The Most Active The greater the shielding, the greater the ability to lose electrons. In general, the bigger the atom, the greater the ability to lose electrons. The more easily it loses electrons to form positive. The most reactive metal on the periodic table is francium. The more vigorously it reacts with other substances. Most reactive metals are at the top while the. Which Metal Is The Most Active.
From brokeasshome.com
Where Are The Most Active Metals Located Periodic Table Worksheet Which Metal Is The Most Active The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. Therefore, metallic character increases going down the table,. The most reactive metal on the periodic table is francium. These are the most reactive metals and readily lose electrons to form cations.. Which Metal Is The Most Active.
From exofshwix.blob.core.windows.net
Activity Table Of Metals at Lou Huey blog Which Metal Is The Most Active Most reactive metals are at the top while the least reactive metals at the bottom. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. The more vigorously it reacts with other substances. They are found in groups ia (alkali metals). Which Metal Is The Most Active.
From selftution.com
Reactivity Series of Metals and Nonmetals » Selftution Which Metal Is The Most Active Therefore, metallic character increases going down the table,. The more vigorously it reacts with other substances. The greater the shielding, the greater the ability to lose electrons. The most reactive metal on the periodic table is francium. They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. 9 rows in general, the more reactive. Which Metal Is The Most Active.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download Which Metal Is The Most Active These are the most reactive metals and readily lose electrons to form cations. For any two metals in the series, the metal placed higher in the series. The more vigorously it reacts with other substances. The greater the shielding, the greater the ability to lose electrons. They are found in groups ia (alkali metals) and iia (some alkaline earth metals). Which Metal Is The Most Active.
From www.freeexamacademy.com
Metals Free Exam Academy Which Metal Is The Most Active For any two metals in the series, the metal placed higher in the series. The more vigorously it reacts with other substances. Should this element have the most or least negative value for its standard reduction potential? The most reactive metal on the periodic table is francium. The most active metals are so reactive that they readily combine with the. Which Metal Is The Most Active.
From engineeringlearner.com
Types of Metals and Their Uses [with Pictures] Engineering Learner Which Metal Is The Most Active Which alkali metal should be the most active? These are the most reactive metals and readily lose electrons to form cations. The most reactive metal on the periodic table is francium. Should this element have the most or least negative value for its standard reduction potential? The greater the shielding, the greater the ability to lose electrons. Therefore, metallic character. Which Metal Is The Most Active.
From sciencenotes.org
What Is the Most Reactive Metal? Most Reactive Element? Which Metal Is The Most Active These are the most reactive metals and readily lose electrons to form cations. Therefore, metallic character increases going down the table,. For any two metals in the series, the metal placed higher in the series. Should this element have the most or least negative value for its standard reduction potential? The more vigorously it reacts with other substances. In general,. Which Metal Is The Most Active.
From www.sliderbase.com
Properties of Acids Bases Presentation Chemistry Which Metal Is The Most Active The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. The greater the shielding, the greater the ability to lose electrons. In general, the bigger. Which Metal Is The Most Active.
From anordinary-life.blogspot.co.uk
An Ordinary Life Science Cleaning silver Metal Displacement reaction Which Metal Is The Most Active For any two metals in the series, the metal placed higher in the series. The more vigorously it reacts with other substances. They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. Most reactive metals are at the top while the least reactive metals at the bottom. The more easily it loses electrons to. Which Metal Is The Most Active.
From kaiya-has-douglas.blogspot.com
Which of the Following Elements Has the Greatest Metallic Character Which Metal Is The Most Active They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. Most reactive metals are at the top while the least reactive metals at the bottom. The more vigorously it reacts with other substances. Therefore, metallic character increases going down the table,. In general, the bigger the atom, the greater the ability to lose electrons.. Which Metal Is The Most Active.
From byjus.com
Reactivity Series Reactivity Series of Metals Chart, Features, Uses Which Metal Is The Most Active The most reactive metal on the periodic table is francium. The more vigorously it reacts with other substances. Therefore, metallic character increases going down the table,. For any two metals in the series, the metal placed higher in the series. Should this element have the most or least negative value for its standard reduction potential? The more easily it loses. Which Metal Is The Most Active.
From www.teachoo.com
Displacement Reaction and Reactivity Series Concepts Which Metal Is The Most Active Which alkali metal should be the most active? Should this element have the most or least negative value for its standard reduction potential? Therefore, metallic character increases going down the table,. The greater the shielding, the greater the ability to lose electrons. These are the most reactive metals and readily lose electrons to form cations. 9 rows in general, the. Which Metal Is The Most Active.
From brokeasshome.com
Which Is The Most Reactive Metal In Periodic Table Of Elements Which Metal Is The Most Active The most reactive metal on the periodic table is francium. Should this element have the most or least negative value for its standard reduction potential? Most reactive metals are at the top while the least reactive metals at the bottom. The more vigorously it reacts with other substances. The greater the shielding, the greater the ability to lose electrons. The. Which Metal Is The Most Active.
From www.jlconline.com
Separating Galvanic Metals JLC Online Which Metal Is The Most Active 9 rows in general, the more reactive a metal is: In general, the bigger the atom, the greater the ability to lose electrons. Most reactive metals are at the top while the least reactive metals at the bottom. Which alkali metal should be the most active? The most reactive metal on the periodic table is francium. The more vigorously it. Which Metal Is The Most Active.
From studylib.net
Periodic Table Worksheet 1. Where are the most active metals Which Metal Is The Most Active The more vigorously it reacts with other substances. These are the most reactive metals and readily lose electrons to form cations. Which alkali metal should be the most active? Most reactive metals are at the top while the least reactive metals at the bottom. Therefore, metallic character increases going down the table,. The most reactive metal on the periodic table. Which Metal Is The Most Active.
From www.periodictableprintable.com
Most Active Metal In Periodic Table 2024 Periodic Table Printable Which Metal Is The Most Active The more vigorously it reacts with other substances. The more easily it loses electrons to form positive. These are the most reactive metals and readily lose electrons to form cations. They are found in groups ia (alkali metals) and iia (some alkaline earth metals) of the. For any two metals in the series, the metal placed higher in the series.. Which Metal Is The Most Active.