Titration Concentration Of Solution . The known solution (titrate) is added in. \ce{naoh}\) is required to reach the end point when titrated. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Suppose that a titration is performed and \(20.70 \: Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
from www.chegg.com
Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. \ce{naoh}\) is required to reach the end point when titrated. The known solution (titrate) is added in. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Suppose that a titration is performed and \(20.70 \:
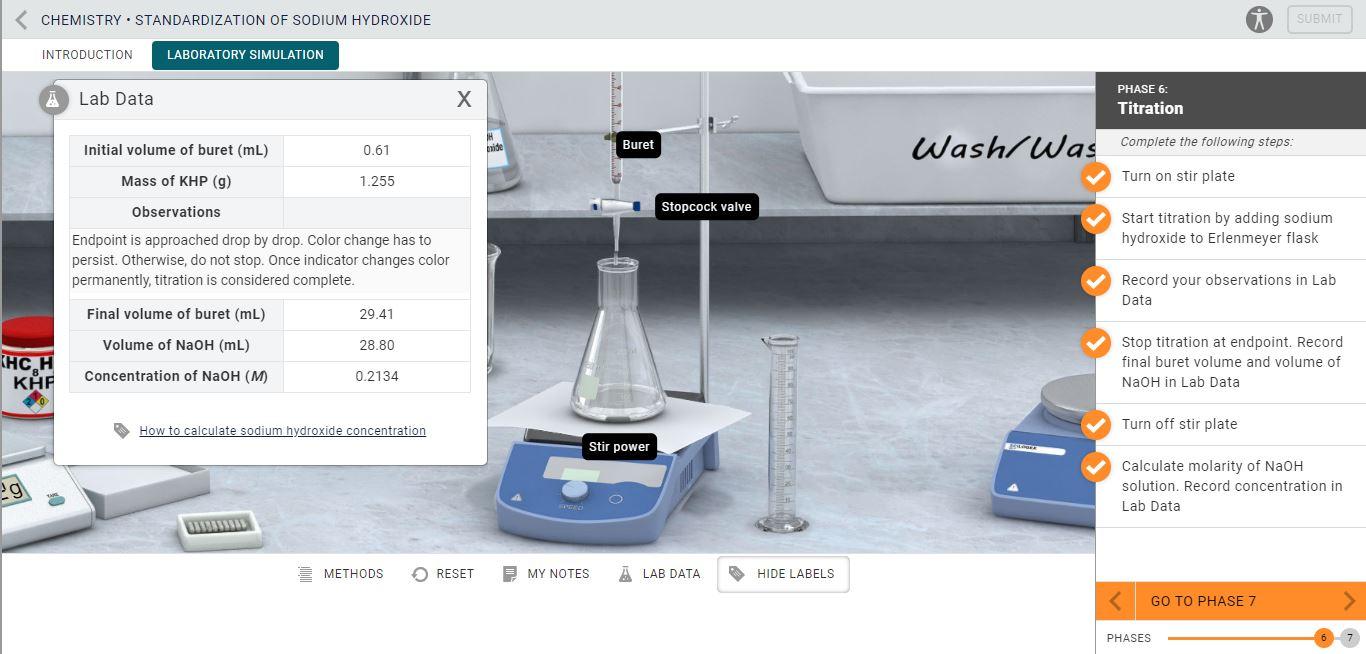
TitrationStandardization of Sodium Hydroxide
Titration Concentration Of Solution \ce{naoh}\) is required to reach the end point when titrated. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Suppose that a titration is performed and \(20.70 \: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The known solution (titrate) is added in. \ce{naoh}\) is required to reach the end point when titrated. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.
From mungfali.com
Acid Base Titration Procedure Titration Concentration Of Solution A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point when titrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of. Titration Concentration Of Solution.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Concentration Of Solution \ce{naoh}\) is required to reach the end point when titrated. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique where. Titration Concentration Of Solution.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Concentration Of Solution Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. A titration is a laboratory technique used to precisely measure molar concentration of an unknown. Titration Concentration Of Solution.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration Concentration Of Solution The known solution (titrate) is added in. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is the slow addition of one solution of a known concentration. Titration Concentration Of Solution.
From mungfali.com
The Titration Of 25 0 Ml Of An Unknown Concentration Of H2so4 Solution 699 Titration Concentration Of Solution The known solution (titrate) is added in. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. A titration is a laboratory technique used to precisely measure molar. Titration Concentration Of Solution.
From chem.libretexts.org
4.5 Concentration of Solutions Chemistry LibreTexts Titration Concentration Of Solution Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to. Titration Concentration Of Solution.
From shelbyscrosbyo.blob.core.windows.net
Titration Method Definition at shelbyscrosbyo blog Titration Concentration Of Solution A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. The known solution (titrate) is added in. \ce{naoh}\) is required to reach the end point when titrated. A titration. Titration Concentration Of Solution.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Titration Concentration Of Solution A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Suppose that a titration is performed and \(20.70 \: Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a technique of determining the. Titration Concentration Of Solution.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Concentration Of Solution Suppose that a titration is performed and \(20.70 \: Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique where a solution of known concentration. Titration Concentration Of Solution.
From saylordotorg.github.io
Solution Concentrations Titration Concentration Of Solution Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The known solution (titrate) is added in. Suppose that a titration is performed and \(20.70 \: Titration is the slow. Titration Concentration Of Solution.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution Titration Concentration Of Solution A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. \ce{naoh}\) is required to reach the end point when titrated. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is the slow addition of one solution of. Titration Concentration Of Solution.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Concentration Of Solution The known solution (titrate) is added in. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique of determining the concentration of. Titration Concentration Of Solution.
From labthink.netlify.app
What is titration and how does it work Titration Concentration Of Solution A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \:. Titration Concentration Of Solution.
From www.alamy.com
Titration experiment, adding chemical of known concentration to test Titration Concentration Of Solution Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Suppose that a titration is performed and \(20.70 \: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique where a. Titration Concentration Of Solution.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Concentration Of Solution Suppose that a titration is performed and \(20.70 \: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. A titration is a laboratory technique used to precisely measure. Titration Concentration Of Solution.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Concentration Of Solution Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. \ce{naoh}\) is required to reach the end point when titrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique where a solution of. Titration Concentration Of Solution.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Concentration Of Solution Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Titration Concentration Of Solution.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Titration Concentration Of Solution \ce{naoh}\) is required to reach the end point when titrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a technique of determining. Titration Concentration Of Solution.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Concentration Of Solution The known solution (titrate) is added in. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to. Titration Concentration Of Solution.
From fity.club
Titration Titration Concentration Of Solution \ce{naoh}\) is required to reach the end point when titrated. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. The known solution (titrate) is added in. Suppose that a titration is performed and \(20.70 \: Titration is the slow addition of one solution of a known concentration (called a. Titration Concentration Of Solution.
From saylordotorg.github.io
AcidBase Titrations Titration Concentration Of Solution Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Suppose that a titration is performed and \(20.70 \: The known solution (titrate) is added in. \ce{naoh}\) is required. Titration Concentration Of Solution.
From www.vrogue.co
The Graphs Labeled A And B Show The Titration Curves vrogue.co Titration Concentration Of Solution A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. \ce{naoh}\) is required to reach the end point when titrated. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration is a technique of determining the concentration of. Titration Concentration Of Solution.
From www.chegg.com
TitrationStandardization of Sodium Hydroxide Titration Concentration Of Solution A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration is a technique of determining the concentration of an unknown solution by using a solution of a. Titration Concentration Of Solution.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 Titration Concentration Of Solution A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point when titrated. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration is the slow addition of one solution of. Titration Concentration Of Solution.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry Titration Concentration Of Solution A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose. Titration Concentration Of Solution.
From www.chegg.com
Solved CHEMISTRY. IDENTIFY WEAK ACID USING TITRATION CURVE A Titration Concentration Of Solution \ce{naoh}\) is required to reach the end point when titrated. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Suppose that a titration is performed and \(20.70 \: The known solution (titrate) is added in. Titration involves the gradual addition of a reagent of known concentration, known as the. Titration Concentration Of Solution.
From www.vrogue.co
Titration Concentration Calculations Complete Lesson vrogue.co Titration Concentration Of Solution A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration Concentration Of Solution.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Titration Concentration Of Solution A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. \ce{naoh}\) is required to reach the end point when titrated. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. The known solution (titrate) is added in. Suppose that a. Titration Concentration Of Solution.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Concentration Of Solution Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Suppose that a titration is performed and \(20.70 \: A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. The known solution (titrate) is added. Titration Concentration Of Solution.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Concentration Of Solution Suppose that a titration is performed and \(20.70 \: Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique where a solution of known concentration. Titration Concentration Of Solution.
From mungfali.com
Acid Base Titration Calculation Titration Concentration Of Solution A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. \ce{naoh}\) is required to reach the end point when titrated. Titration is the slow addition of one solution of. Titration Concentration Of Solution.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic Titration Concentration Of Solution Suppose that a titration is performed and \(20.70 \: Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration is a technique of determining. Titration Concentration Of Solution.
From www.science-revision.co.uk
Titrations Titration Concentration Of Solution A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point when titrated. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. The known solution (titrate) is added in. A. Titration Concentration Of Solution.
From mirjamglessmer.com
Measuring the concentration of dissolved oxygen in sea water Part 3 Titration Concentration Of Solution A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point when titrated. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory. Titration Concentration Of Solution.
From opentextbc.ca
14.7 AcidBase Titrations Chemistry Titration Concentration Of Solution \ce{naoh}\) is required to reach the end point when titrated. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Suppose that a titration is performed. Titration Concentration Of Solution.