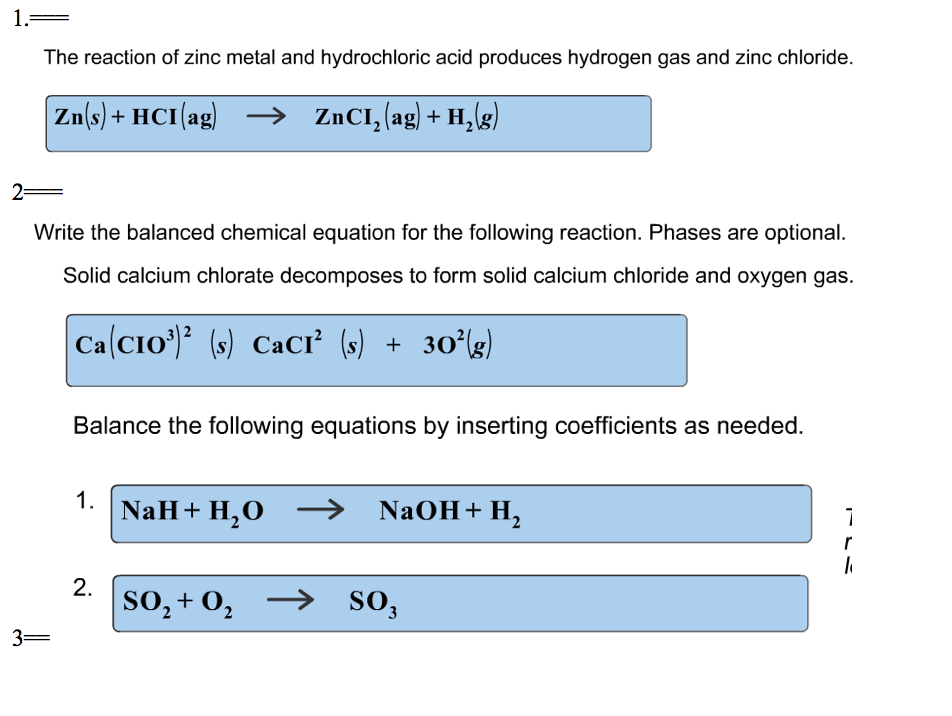
Solid Zinc And Aqueous Hydrochloric Acid . Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. This chemistry video tutorial explains how to predict the products of the single replacement. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Let's imagine we are in the lab. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. Zinc reacts with halogens in the presence of moisture: Zn + cl₂ → zncl₂. We take a piece of metallic zinc and drop it into a beaker that contains dilute. Hydrochloric acid + zinc → zinc chloride +.
from slidesharetrick.blogspot.com
Let's imagine we are in the lab. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Hydrochloric acid + zinc → zinc chloride +. Zn + cl₂ → zncl₂. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. We take a piece of metallic zinc and drop it into a beaker that contains dilute. Acid + metal → salt + hydrogen. Zinc reacts with halogens in the presence of moisture: Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid):
Zinc And Hydrochloric Acid slidesharetrick
Solid Zinc And Aqueous Hydrochloric Acid Acid + metal → salt + hydrogen. Hydrochloric acid + zinc → zinc chloride +. Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zinc reacts with halogens in the presence of moisture: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zn + cl₂ → zncl₂. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. This chemistry video tutorial explains how to predict the products of the single replacement. Acid + metal → salt + hydrogen. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Let's imagine we are in the lab. We take a piece of metallic zinc and drop it into a beaker that contains dilute.
From www.chegg.com
Solved Solid zinc reacts with aqueous hydrochloric acid Solid Zinc And Aqueous Hydrochloric Acid Acid + metal → salt + hydrogen. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. Zinc reacts with halogens in the presence of moisture: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of. Solid Zinc And Aqueous Hydrochloric Acid.
From slideplayer.com
Introduction to Chemical Reactions ppt download Solid Zinc And Aqueous Hydrochloric Acid Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Hydrochloric acid + zinc → zinc chloride +. Acid + metal → salt + hydrogen. This chemistry video tutorial explains how to predict the products of the single replacement. We take a piece. Solid Zinc And Aqueous Hydrochloric Acid.
From slideplayer.com
Chemical Reactions & Equations ppt download Solid Zinc And Aqueous Hydrochloric Acid This chemistry video tutorial explains how to predict the products of the single replacement. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of. Solid Zinc And Aqueous Hydrochloric Acid.
From slideplayer.com
Types of Chemical Reactions and Solution Stoichiometry ppt download Solid Zinc And Aqueous Hydrochloric Acid Acid + metal → salt + hydrogen. Hydrochloric acid + zinc → zinc chloride +. Zn + cl₂ → zncl₂. This chemistry video tutorial explains how to predict the products of the single replacement. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Acids will react with. Solid Zinc And Aqueous Hydrochloric Acid.
From slidesharetrick.blogspot.com
Zinc And Hydrochloric Acid slidesharetrick Solid Zinc And Aqueous Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride +. Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. Zinc reacts with halogens in the presence of moisture: When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): We take a piece of metallic zinc and drop it into. Solid Zinc And Aqueous Hydrochloric Acid.
From oneclass.com
OneClass Hydrogen gas and aqueous zinc chloride are formed by the Solid Zinc And Aqueous Hydrochloric Acid Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. Zinc reacts with halogens in the presence of moisture: Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn (s) + 2hcl (aq) → h2(g). Solid Zinc And Aqueous Hydrochloric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Solid Zinc And Aqueous Hydrochloric Acid Zn + cl₂ → zncl₂. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. This chemistry video tutorial explains how to predict the products of the single replacement. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc. Solid Zinc And Aqueous Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 Solid Zinc And Aqueous Hydrochloric Acid Zn + cl₂ → zncl₂. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. This chemistry video tutorial explains how to predict the. Solid Zinc And Aqueous Hydrochloric Acid.
From www.chegg.com
Solved In this problem, zinc reacts with hydrochloric acid Solid Zinc And Aqueous Hydrochloric Acid Acid + metal → salt + hydrogen. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Learn how zinc metal reacts with aqueous hydrochloric acid to. Solid Zinc And Aqueous Hydrochloric Acid.
From www.numerade.com
SOLVED Aqueous hydrochloric acid (HCl) reacts with solid sodium Solid Zinc And Aqueous Hydrochloric Acid Acid + metal → salt + hydrogen. Hydrochloric acid + zinc → zinc chloride +. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Solid Zinc And Aqueous Hydrochloric Acid.
From www.chegg.com
Solved Zinc metal reacts with aqueous hydrochloric acid to Solid Zinc And Aqueous Hydrochloric Acid Let's imagine we are in the lab. This chemistry video tutorial explains how to predict the products of the single replacement. Zn + cl₂ → zncl₂. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Acids will react with reactive metals, such as. Solid Zinc And Aqueous Hydrochloric Acid.
From slideplayer.com
Reactions in Aqueous Solution ppt download Solid Zinc And Aqueous Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. When zinc metal is submerged into a. Solid Zinc And Aqueous Hydrochloric Acid.
From express.adobe.com
Zinc and Hydrochloric Acid Solid Zinc And Aqueous Hydrochloric Acid Acid + metal → salt + hydrogen. Zinc reacts with halogens in the presence of moisture: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. We take a piece of metallic zinc and drop it into a beaker that contains dilute. This chemistry video tutorial explains how to predict the products of. Solid Zinc And Aqueous Hydrochloric Acid.
From www.slideserve.com
PPT Single Replacement Reactions PowerPoint Presentation, free Solid Zinc And Aqueous Hydrochloric Acid This chemistry video tutorial explains how to predict the products of the single replacement. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq). Solid Zinc And Aqueous Hydrochloric Acid.
From www.chegg.com
Solved Aqueous zinc chloride and hydrogen gas are formed by Solid Zinc And Aqueous Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zinc reacts with halogens in the presence of moisture: Hydrochloric acid + zinc → zinc chloride +. We take a piece of metallic zinc and drop it into a beaker that contains dilute. Acid + metal → salt + hydrogen. Let's imagine we. Solid Zinc And Aqueous Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Solid Zinc And Aqueous Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Zn + cl₂ → zncl₂. Acid + metal → salt + hydrogen. This chemistry video tutorial explains how to predict the. Solid Zinc And Aqueous Hydrochloric Acid.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Solid Zinc And Aqueous Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Hydrochloric acid + zinc → zinc chloride +. We take a piece of metallic zinc and drop it into a beaker that contains dilute. Let's imagine we are in the lab. Acid + metal → salt + hydrogen. This chemistry video tutorial explains. Solid Zinc And Aqueous Hydrochloric Acid.
From www.chegg.com
Solved Solid zinc(II) carbonate reacts with us Hydrochloric Solid Zinc And Aqueous Hydrochloric Acid Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. We take a piece of metallic zinc and drop it into a beaker that contains dilute. Zinc reacts with halogens in the presence of moisture: This chemistry video tutorial explains how to predict the products of the single replacement. Acids. Solid Zinc And Aqueous Hydrochloric Acid.
From express.adobe.com
Zinc and Hydrochloric Acid Solid Zinc And Aqueous Hydrochloric Acid Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. Let's imagine we are in the lab. Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. Zinc reacts with halogens in the presence of moisture: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where. Solid Zinc And Aqueous Hydrochloric Acid.
From studymind.co.uk
Electrolysis of Aqueous Solutions (GCSE Chemistry) Study Mind Solid Zinc And Aqueous Hydrochloric Acid Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. We take a piece of metallic zinc and drop it into a beaker that contains dilute. When zinc metal is submerged into a quantity. Solid Zinc And Aqueous Hydrochloric Acid.
From slideplayer.com
Unit 5 Chemical Reactions Chapter 8 Sec. 1. Objectives Indications of Solid Zinc And Aqueous Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Zinc reacts with halogens in the presence of moisture: This chemistry video tutorial explains how to predict the products of the. Solid Zinc And Aqueous Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science Solid Zinc And Aqueous Hydrochloric Acid Zn + cl₂ → zncl₂. This chemistry video tutorial explains how to predict the products of the single replacement. Let's imagine we are in the lab. Acid + metal → salt + hydrogen. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. When zinc metal is submerged into a. Solid Zinc And Aqueous Hydrochloric Acid.
From express.adobe.com
Zinc and Hydrochloric Acid Solid Zinc And Aqueous Hydrochloric Acid When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): We take a piece of metallic zinc and drop it into a beaker that contains dilute. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. Hydrochloric acid. Solid Zinc And Aqueous Hydrochloric Acid.
From www.numerade.com
SOLVED Text Writing and Balancing Chemical Equations Write balanced Solid Zinc And Aqueous Hydrochloric Acid Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. We take a piece of metallic zinc and drop it into a beaker that. Solid Zinc And Aqueous Hydrochloric Acid.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Solid Zinc And Aqueous Hydrochloric Acid Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. Zn + cl₂ → zncl₂. We take a piece of metallic zinc and drop it into a beaker that contains dilute. Zinc reacts with halogens in the presence of moisture: When zinc metal is submerged into a quantity of aqueous. Solid Zinc And Aqueous Hydrochloric Acid.
From quizlet.com
investigate reactions between dilute hydrochloric and sulfuric acids Solid Zinc And Aqueous Hydrochloric Acid Let's imagine we are in the lab. Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn + cl₂ → zncl₂. Zinc reacts with halogens in the presence of moisture: This chemistry video tutorial explains how to predict the products of the. Solid Zinc And Aqueous Hydrochloric Acid.
From www.numerade.com
SOLVED Text Writing and Balancing Chemical Equations Write balanced Solid Zinc And Aqueous Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride +. Zn + cl₂ → zncl₂. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Let's imagine we are in the lab. We take a piece of metallic zinc and drop it into a beaker that contains dilute. Zinc reacts. Solid Zinc And Aqueous Hydrochloric Acid.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Solid Zinc And Aqueous Hydrochloric Acid Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. This chemistry video tutorial explains how to predict the products of the single replacement. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn + cl₂ → zncl₂. Zinc reacts with halogens. Solid Zinc And Aqueous Hydrochloric Acid.
From www.numerade.com
Consider the chemical reaction that takes place between solid magnesium Solid Zinc And Aqueous Hydrochloric Acid Let's imagine we are in the lab. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. This chemistry video tutorial explains how to predict the products. Solid Zinc And Aqueous Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science Solid Zinc And Aqueous Hydrochloric Acid When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zn + cl₂ → zncl₂. Let's imagine we are in the lab.. Solid Zinc And Aqueous Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0622 Solid Zinc And Aqueous Hydrochloric Acid Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. This chemistry video tutorial explains how to. Solid Zinc And Aqueous Hydrochloric Acid.
From www.numerade.com
SOLVED Zinc metal reacts with aqueous hydrochloric acid to produce a Solid Zinc And Aqueous Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride +. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction. Solid Zinc And Aqueous Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0223 Solid Zinc And Aqueous Hydrochloric Acid Let's imagine we are in the lab. Hydrochloric acid + zinc → zinc chloride +. Zinc reacts with halogens in the presence of moisture: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Acids will react with reactive metals, such as magnesium and. Solid Zinc And Aqueous Hydrochloric Acid.
From warreninstitute.org
Unlock The POWER Of Chemical Reaction Zn + HCl To Zinc + Hydrochloric Acid Solid Zinc And Aqueous Hydrochloric Acid Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride in a single replacement. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Let's imagine we are in the lab. Zinc reacts with halogens in the presence. Solid Zinc And Aqueous Hydrochloric Acid.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Solid Zinc And Aqueous Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn (s) + 2hcl (aq) → h2(g) + zncl2(aq) this. Zinc reacts with halogens in the presence of moisture: Hydrochloric acid + zinc → zinc chloride +. Learn how zinc metal reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride. Solid Zinc And Aqueous Hydrochloric Acid.