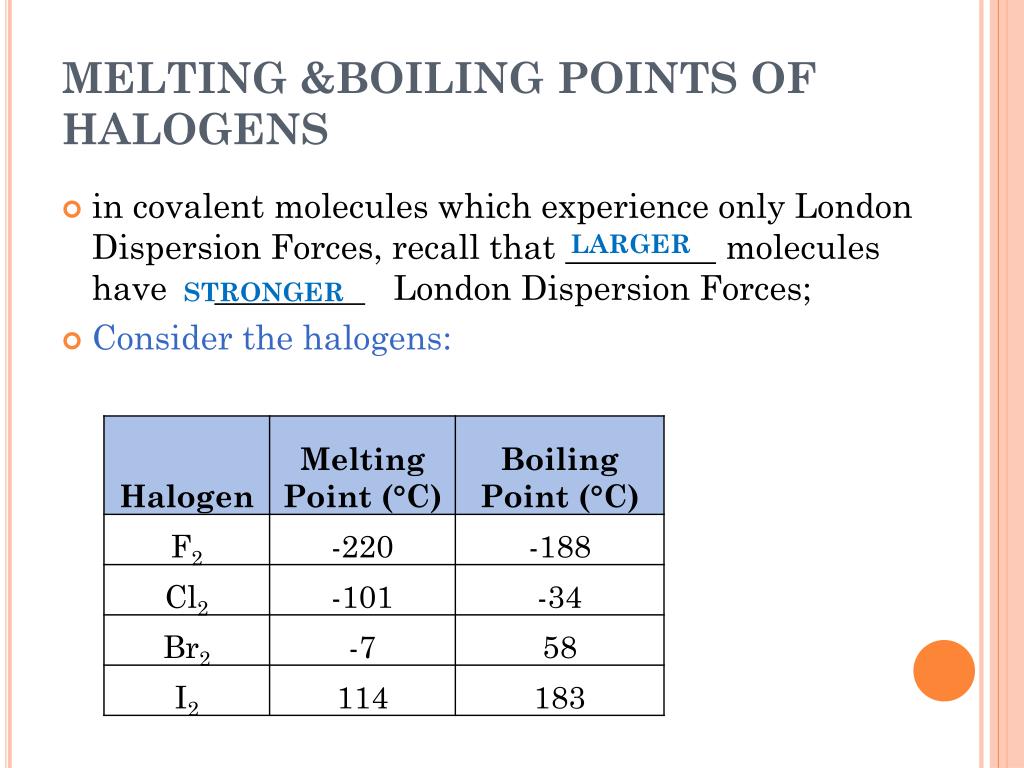
Properties Of Halogen Boiling Point . The size of the molecules increases down the. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. These are typical of a simple covalent substance. This includes their melting points, boiling. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. The melting points (and boiling points) increase with increased relative. Fluorine has the lowest melting and. The halogens have low melting points and low boiling points. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. The melting and boiling points increase down the group because of the van der waals forces. Predict the melting and boiling points of astatine, and its state at room temperature.
from www.slideserve.com
Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. This includes their melting points, boiling. The melting points (and boiling points) increase with increased relative. These are typical of a simple covalent substance. Fluorine has the lowest melting and. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. The melting and boiling points increase down the group because of the van der waals forces. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. Predict the melting and boiling points of astatine, and its state at room temperature.
PPT melting & boiling points PowerPoint Presentation, free download
Properties Of Halogen Boiling Point Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. The melting and boiling points increase down the group because of the van der waals forces. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. The halogens have low melting points and low boiling points. The melting points (and boiling points) increase with increased relative. Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. The size of the molecules increases down the. Fluorine has the lowest melting and. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. Predict the melting and boiling points of astatine, and its state at room temperature. These are typical of a simple covalent substance. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. This includes their melting points, boiling.
From brainly.com
Draw a graph of number of electrons in the halogen molecule against the Properties Of Halogen Boiling Point Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. The melting points (and boiling points) increase with increased relative. Fluorine has the lowest melting and. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling. Properties Of Halogen Boiling Point.
From gampmagazine.weebly.com
Halogen periodic table gampmagazine Properties Of Halogen Boiling Point Predict the melting and boiling points of astatine, and its state at room temperature. Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. These are typical of a simple covalent substance. The halogens have low melting points and low boiling points. It can be seen that there is a regular increase in many of the. Properties Of Halogen Boiling Point.
From newtondesk.com
Halogens (Periodic Table) Properties, Uses, & Facts Properties Of Halogen Boiling Point These are typical of a simple covalent substance. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. The melting and boiling points increase down the group because of the van der waals forces. Sections below cover the trends in atomic radius, electronegativity, electron. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID2942238 Properties Of Halogen Boiling Point This includes their melting points, boiling. Fluorine has the lowest melting and. Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. It can be seen that there is a regular increase in many of the properties of the halogens. Properties Of Halogen Boiling Point.
From slideplayer.com
Organic Chemistry 2 CHEM ppt download Properties Of Halogen Boiling Point Fluorine has the lowest melting and. The melting points (and boiling points) increase with increased relative. The halogens have low melting points and low boiling points. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. The size of the molecules increases down the. The melting and. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Properties Of Halogen Boiling Point Astatine should have a melting point of about 300°c and a boiling point of about 340°c. The melting points (and boiling points) increase with increased relative. These are typical of a simple covalent substance. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine.. Properties Of Halogen Boiling Point.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway C41 ab Halogen physical properties Properties Of Halogen Boiling Point The melting and boiling points increase down the group because of the van der waals forces. Predict the melting and boiling points of astatine, and its state at room temperature. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. Halogens are diatomic molecules in which covalent. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID5949337 Properties Of Halogen Boiling Point The size of the molecules increases down the. Predict the melting and boiling points of astatine, and its state at room temperature. This includes their melting points, boiling. The melting and boiling points increase down the group because of the van der waals forces. It can be seen that there is a regular increase in many of the properties of. Properties Of Halogen Boiling Point.
From chemizi.blogspot.com
Halogen elementsdefinitionpropertiesreactivity and uses. Properties Of Halogen Boiling Point Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. The melting points (and boiling points) increase with increased relative. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. This includes their melting points, boiling. Predict the melting and boiling points of astatine, and its state at room temperature.. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Alkyl halides PowerPoint Presentation, free download ID3480211 Properties Of Halogen Boiling Point This includes their melting points, boiling. The size of the molecules increases down the. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. It can be seen that there is. Properties Of Halogen Boiling Point.
From www.chemistrystudent.com
Group 7 Halogens Boiling Points (ALevel) ChemistryStudent Properties Of Halogen Boiling Point This includes their melting points, boiling. Fluorine has the lowest melting and. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. The halogens have low melting points and low boiling points. The melting points (and boiling points) increase with increased relative. The melting. Properties Of Halogen Boiling Point.
From sciencenotes.org
Halogen Elements List and Facts Properties Of Halogen Boiling Point In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. The halogens have low melting points and low boiling points. These are typical of a simple covalent substance. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. The melting and boiling points increase down the group because. Properties Of Halogen Boiling Point.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 2.2.1 Group 7 (Halogens)翰林国际教育 Properties Of Halogen Boiling Point The melting and boiling points increase down the group because of the van der waals forces. The halogens have low melting points and low boiling points. The size of the molecules increases down the. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. It can be seen that there is a regular increase. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Group 17 The Halogens PowerPoint Presentation, free download Properties Of Halogen Boiling Point The size of the molecules increases down the. The melting points (and boiling points) increase with increased relative. The melting and boiling points increase down the group because of the van der waals forces. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Predict the melting and boiling points of astatine, and its. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Properties Of Halogen Boiling Point Fluorine has the lowest melting and. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. These are typical of a simple covalent substance. The halogens have low melting points and. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT melting & boiling points PowerPoint Presentation, free download Properties Of Halogen Boiling Point Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Predict the melting and boiling points of astatine, and its state. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Properties Of Halogen Boiling Point Fluorine has the lowest melting and. The halogens have low melting points and low boiling points. The size of the molecules increases down the. This includes their melting points, boiling. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. In a covalent bond,. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Properties Of Halogen Boiling Point In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. The halogens have low melting points and low boiling points. Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. These are typical of a simple covalent substance. Fluorine has the lowest melting and. The size of the molecules increases. Properties Of Halogen Boiling Point.
From www.thoughtco.com
Halogen Elements and Properties Properties Of Halogen Boiling Point These are typical of a simple covalent substance. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. The melting points (and boiling points) increase with increased relative. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. The melting and. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT CHAPTER 5 ORGANIC HALOGEN COMPOUNDS PowerPoint Presentation, free Properties Of Halogen Boiling Point It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Predict the melting and boiling points of astatine, and its state at room temperature. These are typical of a simple covalent substance. This includes their melting points, boiling. Halogens are diatomic molecules in which. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1039137 Properties Of Halogen Boiling Point Predict the melting and boiling points of astatine, and its state at room temperature. The melting and boiling points increase down the group because of the van der waals forces. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. These are typical of a simple covalent. Properties Of Halogen Boiling Point.
From slideplayer.com
Chemistry Department, College of Science, King Saud University ppt Properties Of Halogen Boiling Point The melting points (and boiling points) increase with increased relative. The halogens have low melting points and low boiling points. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. The melting and boiling points increase down the group because of the van der waals forces. Sections below cover the trends in atomic radius,. Properties Of Halogen Boiling Point.
From www.slideshare.net
Lesson 1 Group 7 Elements Eam Properties Of Halogen Boiling Point It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. The melting and boiling points increase down the group because of the van der waals forces. The halogens have low. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID5566024 Properties Of Halogen Boiling Point Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. The halogens have low melting points and low boiling points. In a covalent bond, the bonding pair of electrons is attracted to the. Properties Of Halogen Boiling Point.
From courses.lumenlearning.com
Intermolecular Forces Chemistry Properties Of Halogen Boiling Point This includes their melting points, boiling. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. Predict the melting and boiling points of astatine, and its state at room temperature. These. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT AN INTRODUCTION TO THE CHEMISTRY OF HALOGENOALKANES PowerPoint Properties Of Halogen Boiling Point This includes their melting points, boiling. The size of the molecules increases down the. These are typical of a simple covalent substance. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. Fluorine has the lowest melting and. The halogens have low melting points and low boiling points. Predict the melting and boiling points. Properties Of Halogen Boiling Point.
From wwwchem.uwimona.edu.jm
The Chemistry of the Halogens 2 Properties Of Halogen Boiling Point The halogens have low melting points and low boiling points. The melting points (and boiling points) increase with increased relative. The melting and boiling points increase down the group because of the van der waals forces. Predict the melting and boiling points of astatine, and its state at room temperature. Halogens are diatomic molecules in which covalent bonds are formed. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2319114 Properties Of Halogen Boiling Point Astatine should have a melting point of about 300°c and a boiling point of about 340°c. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. The melting points (and boiling points) increase with increased relative. The size of the molecules increases down the. Sections below cover the trends in atomic radius, electronegativity, electron. Properties Of Halogen Boiling Point.
From www.researchgate.net
Some properties of the Halogens Download Table Properties Of Halogen Boiling Point Astatine should have a melting point of about 300°c and a boiling point of about 340°c. The size of the molecules increases down the. Predict the melting and boiling points of astatine, and its state at room temperature. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17. Properties Of Halogen Boiling Point.
From slideplayer.com
Melting and Boiling points? ppt download Properties Of Halogen Boiling Point This includes their melting points, boiling. The melting and boiling points increase down the group because of the van der waals forces. These are typical of a simple covalent substance. Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. The melting points (and boiling points) increase with increased relative. The halogens have low melting points. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Properties Of Halogen Boiling Point Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. Fluorine has the lowest melting and. Predict the melting and boiling points of astatine, and its state at room temperature. This includes their melting points, boiling. It can be seen that there is a regular increase in. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation ID3005113 Properties Of Halogen Boiling Point In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. The halogens have low melting points and low boiling points. The size of the molecules increases down the. It can be seen that there is a regular increase in many. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Properties Of Halogen Boiling Point The melting and boiling points increase down the group because of the van der waals forces. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. These are typical of a simple covalent substance. Fluorine has the lowest melting and. The melting points (and boiling points) increase. Properties Of Halogen Boiling Point.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Properties Of Halogen Boiling Point Fluorine has the lowest melting and. The halogens have low melting points and low boiling points. Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals. The size of the molecules increases down the. In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either. These are typical of a simple. Properties Of Halogen Boiling Point.
From chem.libretexts.org
Group 17 Physical Properties of the Halogens Chemistry LibreTexts Properties Of Halogen Boiling Point These are typical of a simple covalent substance. The melting points (and boiling points) increase with increased relative. The melting and boiling points increase down the group because of the van der waals forces. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine.. Properties Of Halogen Boiling Point.