Half Life Equation For First Order . T ½ = [a o] / 2k. Rate = k [n 2 o 5] with time We can determine the half life for a first order reaction from a graph of concentration against time. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: For a second order reaction 2a products or a. What is its rate constant? T ½ = 0.693 / k.
from www.chegg.com
Rate = k [n 2 o 5] with time For a first order reaction a products , rate = k [a]: What is its rate constant? For a second order reaction 2a products or a. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: We can determine the half life for a first order reaction from a graph of concentration against time. T ½ = 0.693 / k. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order:
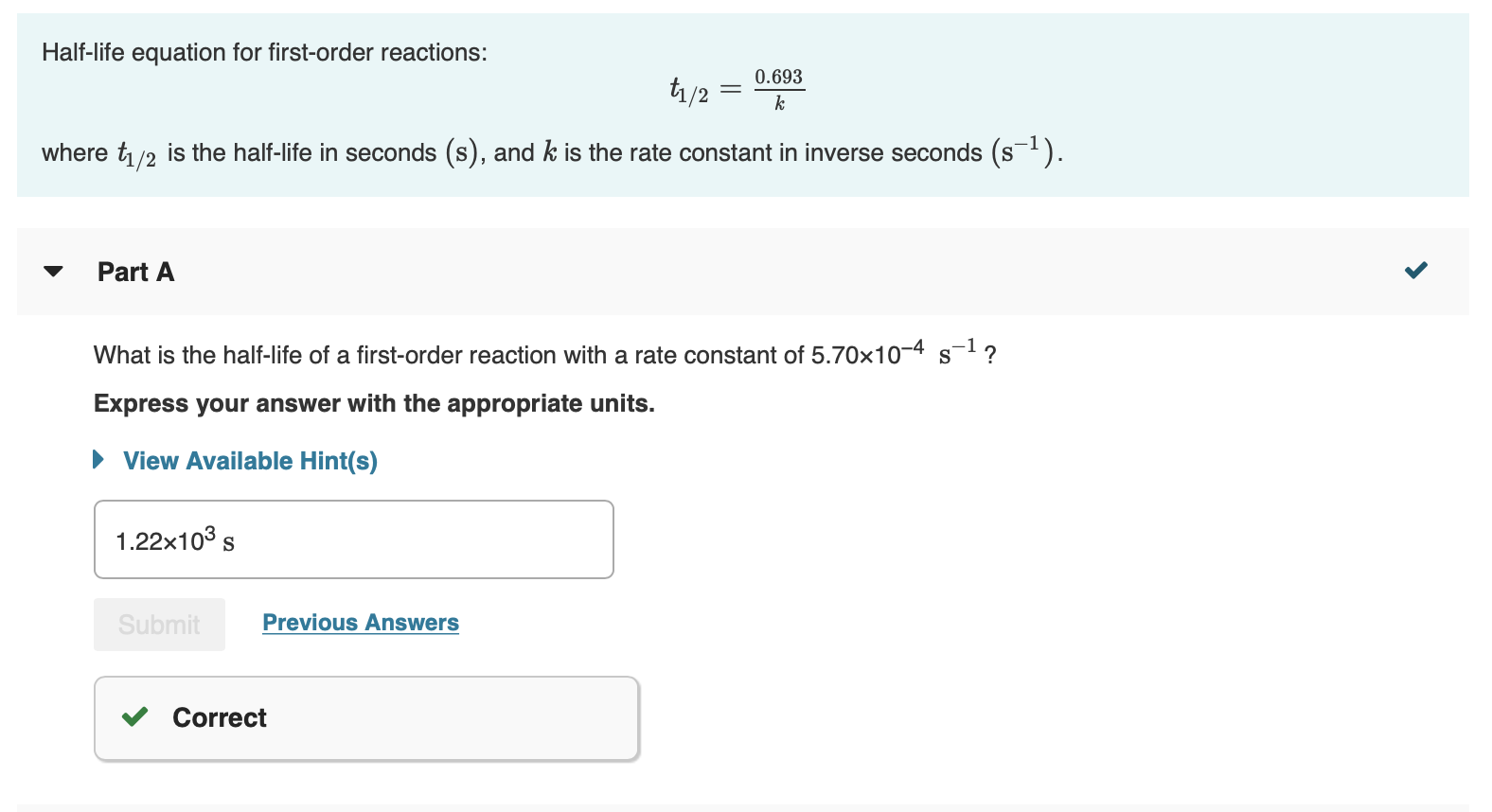
Solved Halflife equation for firstorder reactions t1/2
Half Life Equation For First Order What is its rate constant? T ½ = 0.693 / k. We can determine the half life for a first order reaction from a graph of concentration against time. The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: For a first order reaction a products , rate = k [a]: What is its rate constant? For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. Rate = k [n 2 o 5] with time For a second order reaction 2a products or a. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\]
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation For First Order For a second order reaction 2a products or a. T ½ = [a o] / 2k. What is its rate constant? \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] For a zero order reaction a products , rate = k: The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order:. Half Life Equation For First Order.
From yoursoundboard.blogspot.com
half life formula for first order reaction Ashlee Manley Half Life Equation For First Order T ½ = [a o] / 2k. We can determine the half life for a first order reaction from a graph of concentration against time. T ½ = 0.693 / k. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] For a second order reaction 2a products or a. For a first order reaction a products , rate =. Half Life Equation For First Order.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Half Life Equation For First Order Rate = k [n 2 o 5] with time \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: We can determine the half life for a first order reaction from a graph of concentration against time. For a second order. Half Life Equation For First Order.
From www.chegg.com
Solved Halflife equation for firstorder reactions t1/2 Half Life Equation For First Order T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: Rate = k [n 2 o 5] with time For a second order reaction 2a products or a. What is its rate constant? The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: For. Half Life Equation For First Order.
From www.youtube.com
Determine the halflife of a first order reaction YouTube Half Life Equation For First Order T ½ = [a o] / 2k. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: What is its rate constant? For a first order reaction a products , rate = k [a]: For a zero order reaction a products ,. Half Life Equation For First Order.
From byjus.com
How to calculate the half life of a first order reaction Half Life Equation For First Order For a second order reaction 2a products or a. For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] For a zero order reaction a products , rate = k: What is its rate constant? Rate = k [n 2 o 5]. Half Life Equation For First Order.
From slidesharenow.blogspot.com
Half Life Equation First Order slideshare Half Life Equation For First Order \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] For a zero order reaction a products , rate = k: For a second order reaction 2a products or a. For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. T ½ = [a o] / 2k. The decomposition of dinitrogen pentoxide,. Half Life Equation For First Order.
From lauranfryer.blogspot.com
half life formula for first order reaction Lauran Fryer Half Life Equation For First Order The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: For a zero order reaction a products , rate = k: T ½ = 0.693 / k. For a second order reaction 2a products or a. What is its rate constant? T ½ = [a o] / 2k. Rate = k [n. Half Life Equation For First Order.
From www.numerade.com
SOLVEDPart C PLEASE HELP Halflife equation for firstorder reactions Half Life Equation For First Order For a second order reaction 2a products or a. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: Rate = k [n 2 o 5] with time T ½ = 0.693 / k. The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order:. Half Life Equation For First Order.
From www.youtube.com
Halflife of a firstorder reaction AP Chemistry Khan Half Life Equation For First Order \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: T ½ = 0.693 / k. We can determine the half life for. Half Life Equation For First Order.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation For First Order T ½ = [a o] / 2k. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] We can determine the half life for a first order reaction from a graph of concentration against time. For a second order reaction 2a products or a. Rate = k [n 2 o 5] with time For a first order reaction a products. Half Life Equation For First Order.
From www.slideserve.com
PPT Concepts of Pharmacology Half Life Calculation PowerPoint Half Life Equation For First Order The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] What is its rate constant? T ½ = [a o] / 2k. We can determine the half life for a first order reaction from a graph of concentration against time. For a. Half Life Equation For First Order.
From general.chemistrysteps.com
FirstOrder Reactions Chemistry Steps Half Life Equation For First Order For a second order reaction 2a products or a. Rate = k [n 2 o 5] with time For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. What is its rate constant? We can determine the half life for a first order reaction from a graph of concentration against time. The. Half Life Equation For First Order.
From www.youtube.com
First Order HalfLife Derivation YouTube Half Life Equation For First Order For a first order reaction a products , rate = k [a]: The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: Rate = k [n 2 o 5] with time For a second order reaction 2a products or a. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] We can. Half Life Equation For First Order.
From www.chegg.com
Solved Part 3 The Half Life Equation Let's make a Half Life Equation For First Order T ½ = 0.693 / k. For a second order reaction 2a products or a. The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: T ½ = [a. Half Life Equation For First Order.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID1306821 Half Life Equation For First Order Rate = k [n 2 o 5] with time For a second order reaction 2a products or a. For a zero order reaction a products , rate = k: The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] We can determine. Half Life Equation For First Order.
From www.numerade.com
SOLVED Halflife equation for firstorder reactions t1/2 = 0.693/k Half Life Equation For First Order \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] T ½ = 0.693 / k. For a second order reaction 2a products or a. For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: We can determine the half life for a first order reaction from. Half Life Equation For First Order.
From www.tessshebaylo.com
Derive An Integrated Rate Equation For Constant The First Order Half Life Equation For First Order Rate = k [n 2 o 5] with time \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: We can determine the half life for a first order reaction from a graph of concentration against time.. Half Life Equation For First Order.
From mapleschilling.blogspot.com
half life formula physics Maple Schilling Half Life Equation For First Order We can determine the half life for a first order reaction from a graph of concentration against time. For a second order reaction 2a products or a. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: For a first order reaction. Half Life Equation For First Order.
From lauranfryer.blogspot.com
half life formula for first order reaction Lauran Fryer Half Life Equation For First Order \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] T ½ = 0.693 / k. T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: What is its rate constant? For a second order reaction 2a products or a. The decomposition of dinitrogen pentoxide, n 2 o 5, to form. Half Life Equation For First Order.
From www.vrogue.co
Finding Half Life For A First Order Reaction Science vrogue.co Half Life Equation For First Order T ½ = 0.693 / k. For a zero order reaction a products , rate = k: We can determine the half life for a first order reaction from a graph of concentration against time. The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: \ [\ln\dfrac { [\textrm a]_0} { [\textrm. Half Life Equation For First Order.
From socratic.org
What is a first order half life? Socratic Half Life Equation For First Order For a first order reaction a products , rate = k [a]: T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: Rate = k [n 2 o 5] with time \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] T ½ = 0.693 / k. We can determine the half. Half Life Equation For First Order.
From www.youtube.com
Half Life Equation Derivation YouTube Half Life Equation For First Order For a second order reaction 2a products or a. The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: T ½ = 0.693 / k. T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: For a zero order reaction a products ,. Half Life Equation For First Order.
From lavelle.chem.ucla.edu
halflife CHEMISTRY COMMUNITY Half Life Equation For First Order For a first order reaction a products , rate = k [a]: We can determine the half life for a first order reaction from a graph of concentration against time. What is its rate constant? For a second order reaction 2a products or a. T ½ = 0.693 / k. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\]. Half Life Equation For First Order.
From study.com
Solving for FirstOrder given HalfLife Chemistry Half Life Equation For First Order T ½ = 0.693 / k. Rate = k [n 2 o 5] with time \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: For a zero order reaction a products , rate = k: For a second order reaction 2a. Half Life Equation For First Order.
From ar.inspiredpencil.com
Half Life Equation Half Life Equation For First Order T ½ = 0.693 / k. We can determine the half life for a first order reaction from a graph of concentration against time. The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: For a zero order reaction a products , rate = k: \ [\ln\dfrac { [\textrm a]_0} { [\textrm. Half Life Equation For First Order.
From haipernews.com
How To Calculate Half Life Algebra Haiper Half Life Equation For First Order T ½ = 0.693 / k. For a second order reaction 2a products or a. For a first order reaction a products , rate = k [a]: \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: T ½ = [a o]. Half Life Equation For First Order.
From www.chegg.com
Solved Halflife equation for firstorder reactions t/2 = Half Life Equation For First Order What is its rate constant? For a second order reaction 2a products or a. Rate = k [n 2 o 5] with time \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] For a zero order reaction a products , rate = k: For a first order reaction a products , rate = k [a]: The decomposition of dinitrogen. Half Life Equation For First Order.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation For First Order For a second order reaction 2a products or a. Rate = k [n 2 o 5] with time We can determine the half life for a first order reaction from a graph of concentration against time. For a zero order reaction a products , rate = k: T ½ = 0.693 / k. What is its rate constant? \ [\ln\dfrac. Half Life Equation For First Order.
From www.chegg.com
Solved Halflife equation for firstorder reactions The Half Life Equation For First Order \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] T ½ = 0.693 / k. The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: T ½ = [a o] / 2k. What is its rate constant? Rate = k [n 2 o 5] with time For a first order reaction. Half Life Equation For First Order.
From slidesharenow.blogspot.com
Half Life Equation First Order slideshare Half Life Equation For First Order The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: T ½ = 0.693 / k. What is its rate constant? We can determine the half life for a first order reaction from a graph of concentration against time. For a second order reaction 2a products or a. Rate = k [n. Half Life Equation For First Order.
From www.slideserve.com
PPT Chemical Chapter 14 PowerPoint Presentation, free Half Life Equation For First Order The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: For a second order reaction 2a products or a. T ½ = [a o] / 2k. We can determine the half life for a first order reaction from a graph of concentration against time. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt. Half Life Equation For First Order.
From www.slideserve.com
PPT Chemical Chapter 14 PowerPoint Presentation, free Half Life Equation For First Order For a first order reaction a products , rate = k [a]: T ½ = [a o] / 2k. \ [\ln\dfrac { [\textrm a]_0} { [\textrm a]}=kt \label {21.4.1}\] T ½ = 0.693 / k. For a zero order reaction a products , rate = k: Rate = k [n 2 o 5] with time The decomposition of dinitrogen pentoxide,. Half Life Equation For First Order.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Half Life Equation For First Order For a second order reaction 2a products or a. The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: We can determine the half life for a first order reaction from a graph of concentration against time. For a first order reaction a products , rate = k [a]: T ½ =. Half Life Equation For First Order.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation For First Order For a first order reaction a products , rate = k [a]: The decomposition of dinitrogen pentoxide, n 2 o 5, to form dinitrogen oxide and oxygen is first order: We can determine the half life for a first order reaction from a graph of concentration against time. For a zero order reaction a products , rate = k: T. Half Life Equation For First Order.