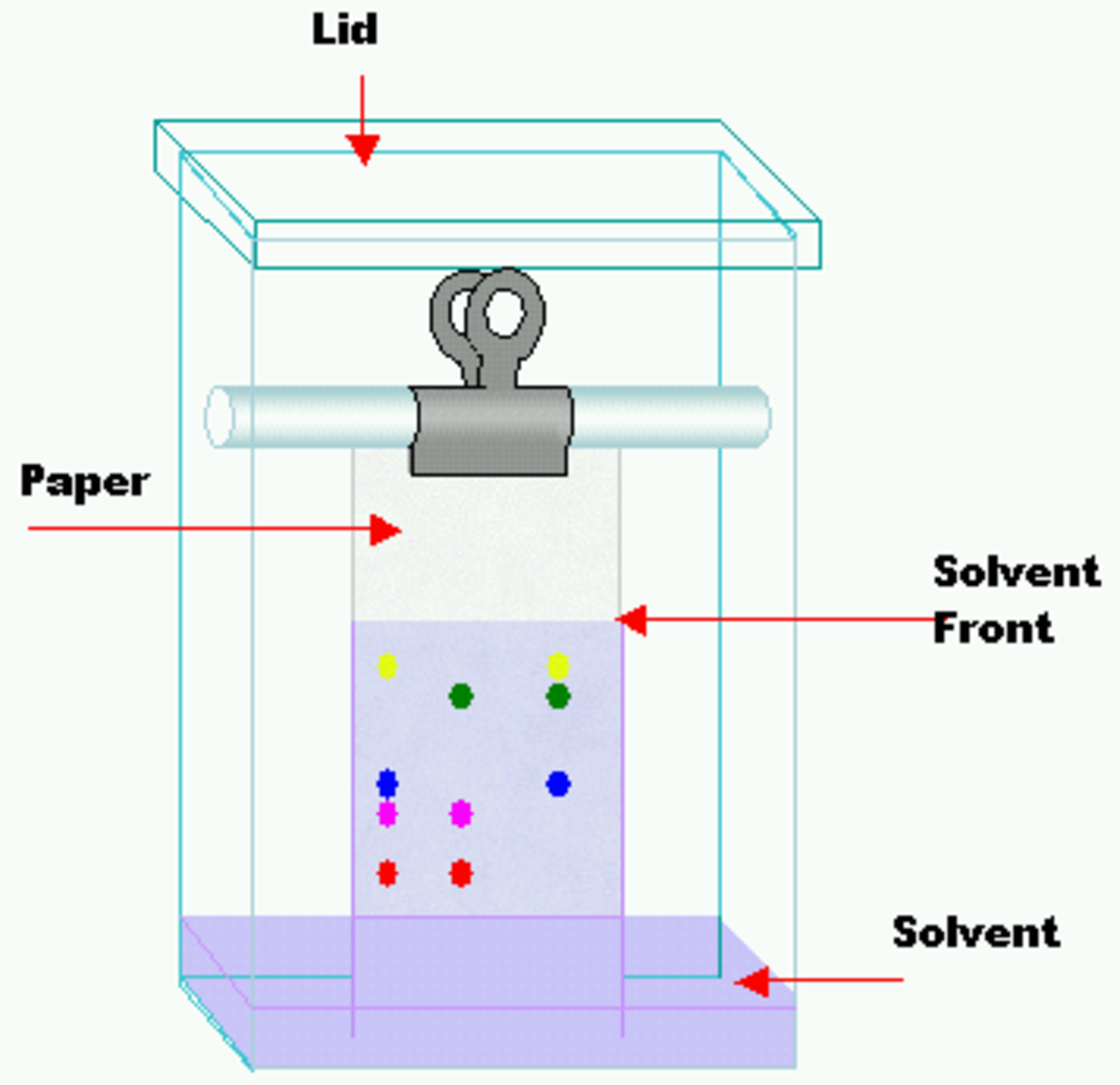
Difference Between Filtration Distillation And Chromatography . Evaporation removes a liquid from a solution to leave a solid. Evaporation removes a liquid from a solution to leave a solid. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass through a filter. Distillation takes advantage of differences in boiling points. Chromatography involves solvent separation on a solid medium. There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Chromatography involves solvent separation on a solid medium. Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate components based on their. The vapor contains a larger proportion of the. The method chosen depends upon the type of mixture. While they share the common goal of separation, chromatography and distillation differ in their principles, applications, and effectiveness. Distillation takes advantage of differences in boiling points.
from joiuvmsyq.blob.core.windows.net
Evaporation removes a liquid from a solution to leave a solid. Chromatography involves solvent separation on a solid medium. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation takes advantage of differences in boiling points. The method chosen depends upon the type of mixture. The vapor contains a larger proportion of the. While they share the common goal of separation, chromatography and distillation differ in their principles, applications, and effectiveness. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass through a filter. Distillation takes advantage of differences in boiling points.
Chromatography Definition Applications at Geneva Atkins blog
Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. Chromatography involves solvent separation on a solid medium. Evaporation removes a liquid from a solution to leave a solid. Chromatography involves solvent separation on a solid medium. Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate components based on their. The vapor contains a larger proportion of the. While they share the common goal of separation, chromatography and distillation differ in their principles, applications, and effectiveness. There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. The method chosen depends upon the type of mixture. Evaporation removes a liquid from a solution to leave a solid. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: Distillation takes advantage of differences in boiling points. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass through a filter. Distillation takes advantage of differences in boiling points.
From slideplayer.com
All About Matter SC2. Obtain, evaluate, and communicate information about the chemical and Difference Between Filtration Distillation And Chromatography Chromatography involves solvent separation on a solid medium. There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation takes advantage of differences in boiling points. Distillation takes advantage of differences in boiling points. While they share the common goal of separation, chromatography and distillation differ in their principles, applications, and effectiveness. Distillation is the selective. Difference Between Filtration Distillation And Chromatography.
From www.studypool.com
SOLUTION Distillation and chromatography Studypool Difference Between Filtration Distillation And Chromatography In general, the composition of the vapor above a liquid mixture differs from that of the liquid: Distillation takes advantage of differences in boiling points. Chromatography involves solvent separation on a solid medium. Evaporation removes a liquid from a solution to leave a solid. Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used. Difference Between Filtration Distillation And Chromatography.
From pediaa.com
Difference Between Distillation and Extraction Definition, Technique, Different Types Difference Between Filtration Distillation And Chromatography Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate components based on their. Distillation takes advantage of differences in boiling points. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a. Difference Between Filtration Distillation And Chromatography.
From www.pinterest.co.uk
Separation techniques filtration, distillation, chromatography, crystallization and more Difference Between Filtration Distillation And Chromatography Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass through a filter. Distillation takes advantage of differences in boiling points. In general, the composition of the vapor above a liquid mixture differs. Difference Between Filtration Distillation And Chromatography.
From www.teachoo.com
What are the Properties of Mixtures? Teachoo Concepts Difference Between Filtration Distillation And Chromatography The vapor contains a larger proportion of the. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: Chromatography involves solvent separation on a solid medium. Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate components based on their. Evaporation removes a liquid from. Difference Between Filtration Distillation And Chromatography.
From www.priyamstudycentre.com
Gel Permeation Chromatography GPC Instrument Difference Between Filtration Distillation And Chromatography In general, the composition of the vapor above a liquid mixture differs from that of the liquid: The vapor contains a larger proportion of the. Chromatography involves solvent separation on a solid medium. Chromatography involves solvent separation on a solid medium. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the. Difference Between Filtration Distillation And Chromatography.
From www.pinterest.com
Mixtures a) Define Atoms, Elements, Mixtures and Compounds b) Difference between compounds and Difference Between Filtration Distillation And Chromatography Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass through a filter. Chromatography involves solvent separation on a solid medium. The method chosen depends upon the type of mixture. There are different. Difference Between Filtration Distillation And Chromatography.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Difference Between Filtration Distillation And Chromatography There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: Evaporation removes a liquid from a solution to leave a solid. The method chosen depends upon the type of mixture. Distillation takes advantage of differences in boiling points. Chromatography. Difference Between Filtration Distillation And Chromatography.
From www.differencebetween.com
Difference Between Filtration and Purification Compare the Difference Between Similar Terms Difference Between Filtration Distillation And Chromatography Chromatography involves solvent separation on a solid medium. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass through a filter. Distillation is the selective boiling and subsequent condensation of a component in. Difference Between Filtration Distillation And Chromatography.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: The method chosen depends upon the type of mixture. Chromatography involves solvent separation on a solid medium. Distillation takes advantage of differences in boiling points. There are different ways to separate mixtures,. Difference Between Filtration Distillation And Chromatography.
From www.youtube.com
How to separate Mixtures filtration, distillation, sublimation and paper chromatography. YouTube Difference Between Filtration Distillation And Chromatography Chromatography involves solvent separation on a solid medium. Distillation takes advantage of differences in boiling points. Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate components based on their. The method chosen depends upon the type of mixture. Distillation takes advantage of differences in boiling points. Chromatography involves solvent separation on. Difference Between Filtration Distillation And Chromatography.
From studylib.net
Chromatography and distillation Difference Between Filtration Distillation And Chromatography Distillation takes advantage of differences in boiling points. Chromatography involves solvent separation on a solid medium. Chromatography involves solvent separation on a solid medium. Evaporation removes a liquid from a solution to leave a solid. There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. The method chosen depends upon the type of mixture. The vapor. Difference Between Filtration Distillation And Chromatography.
From ceddsdrp.blob.core.windows.net
Filtration And Distillation Process at Elvia Archibald blog Difference Between Filtration Distillation And Chromatography The method chosen depends upon the type of mixture. Distillation takes advantage of differences in boiling points. Distillation takes advantage of differences in boiling points. The vapor contains a larger proportion of the. Chromatography involves solvent separation on a solid medium. While they share the common goal of separation, chromatography and distillation differ in their principles, applications, and effectiveness. Evaporation. Difference Between Filtration Distillation And Chromatography.
From www.slideserve.com
PPT CHEMISTRY I PowerPoint Presentation, free download ID3104697 Difference Between Filtration Distillation And Chromatography Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate components based on their. Distillation takes advantage of differences in boiling points. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. Difference Between Filtration Distillation And Chromatography.
From www.scribd.com
Separating Mixtures Filtration, Evaporation, Distillation, and Chromatography PDF Difference Between Filtration Distillation And Chromatography The method chosen depends upon the type of mixture. There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation takes advantage of differences in boiling points. Chromatography involves solvent separation on a solid medium. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: Chromatography involves solvent separation. Difference Between Filtration Distillation And Chromatography.
From byjus.com
What is the difference between distillation, distillation under reduced pressure and steam Difference Between Filtration Distillation And Chromatography Distillation takes advantage of differences in boiling points. Chromatography involves solvent separation on a solid medium. While they share the common goal of separation, chromatography and distillation differ in their principles, applications, and effectiveness. Distillation takes advantage of differences in boiling points. The vapor contains a larger proportion of the. Evaporation removes a liquid from a solution to leave a. Difference Between Filtration Distillation And Chromatography.
From www.transtutors.com
(Solved) Simple distillation, Fractional distillation, Gas Chromatography... (1 Answer Difference Between Filtration Distillation And Chromatography Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate components based on their. Chromatography involves solvent separation on a solid medium. The method chosen depends upon the type of mixture. Evaporation removes a liquid from a solution to leave a solid. While they share the common goal of separation, chromatography and. Difference Between Filtration Distillation And Chromatography.
From 12014dorcascharityng.weebly.com
FILTRATION,EVAPORATION,DISTILLATION AND PAPER CHROMATOGRAPHY 3E1 Dorcas (4) Difference Between Filtration Distillation And Chromatography There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation takes advantage of differences in boiling points. Chromatography involves solvent separation on a solid medium. Chromatography involves solvent separation on a solid medium. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: The vapor contains a larger. Difference Between Filtration Distillation And Chromatography.
From video.yckmc.edu.hk
Chemistry tutorialCh621Filtration, crystallization, simple distillation and fractional Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. Distillation takes advantage of differences in boiling points. Chromatography involves solvent separation on a solid medium. While they share the common goal of separation, chromatography and distillation differ in their principles, applications, and effectiveness. The method chosen depends upon the type of mixture. There are different ways to separate. Difference Between Filtration Distillation And Chromatography.
From www.studypool.com
SOLUTION Distillation and chromatography Studypool Difference Between Filtration Distillation And Chromatography While they share the common goal of separation, chromatography and distillation differ in their principles, applications, and effectiveness. Chromatography involves solvent separation on a solid medium. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: Distillation takes advantage of differences in boiling points. Distillation takes advantage of differences in boiling points. Evaporation. Difference Between Filtration Distillation And Chromatography.
From www.youtube.com
GCSE Chemistry 19 Which Separation Technique? Chromatography? Distillation? Filtration? YouTube Difference Between Filtration Distillation And Chromatography Chromatography involves solvent separation on a solid medium. The vapor contains a larger proportion of the. Evaporation removes a liquid from a solution to leave a solid. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing. Difference Between Filtration Distillation And Chromatography.
From www.chemicals.co.uk
What’s the Difference Between Distilled & Ultrapure Water? The Chemistry Blog Difference Between Filtration Distillation And Chromatography Chromatography involves solvent separation on a solid medium. Distillation takes advantage of differences in boiling points. The method chosen depends upon the type of mixture. Evaporation removes a liquid from a solution to leave a solid. Distillation takes advantage of differences in boiling points. In general, the composition of the vapor above a liquid mixture differs from that of the. Difference Between Filtration Distillation And Chromatography.
From www.slideserve.com
PPT Distillation & Chromatography PowerPoint Presentation, free download ID1964738 Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate components based on their. Evaporation removes a liquid from a solution to leave a solid. There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation takes. Difference Between Filtration Distillation And Chromatography.
From ar.inspiredpencil.com
Decantation Diagram Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. Evaporation removes a liquid from a solution to leave a solid. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass through. Difference Between Filtration Distillation And Chromatography.
From www.slideserve.com
PPT Distillation and Chromatography PowerPoint Presentation, free download ID9351670 Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. Chromatography involves solvent separation on a solid medium. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass through a filter. While. Difference Between Filtration Distillation And Chromatography.
From www.pinterest.co.uk
GCSE OCR CHEMISTRY Elements Compounds and Mixture Chromatography Complete Re… Compounds Difference Between Filtration Distillation And Chromatography Chromatography involves solvent separation on a solid medium. While they share the common goal of separation, chromatography and distillation differ in their principles, applications, and effectiveness. There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. The method chosen depends upon the type of mixture. Evaporation removes a liquid from a solution to leave a solid.. Difference Between Filtration Distillation And Chromatography.
From joiuvmsyq.blob.core.windows.net
Chromatography Definition Applications at Geneva Atkins blog Difference Between Filtration Distillation And Chromatography There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. The vapor contains a larger proportion of the. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass through. Difference Between Filtration Distillation And Chromatography.
From www.slideserve.com
PPT Methods of Separating Mixtures PowerPoint Presentation, free download ID5709613 Difference Between Filtration Distillation And Chromatography While they share the common goal of separation, chromatography and distillation differ in their principles, applications, and effectiveness. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid. Difference Between Filtration Distillation And Chromatography.
From aimhigh.space
Distillation and Chromatography AimHigh! Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. The vapor contains a larger proportion of the. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass through a filter. Chromatography. Difference Between Filtration Distillation And Chromatography.
From www.youtube.com
Chromatography Types, Retention Factor, Filtration, Distillation, Recrystallization Orgo YouTube Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. The method chosen depends upon the type of mixture. The vapor contains a larger proportion of the. Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate components based on their. Distillation takes advantage of differences in boiling points. Evaporation removes. Difference Between Filtration Distillation And Chromatography.
From primaryleap.co.uk
Chemistry Separating Mixtures Level 1 activity for kids PrimaryLeap.co.uk Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. Distillation takes advantage of differences in boiling points. Distillation takes advantage of differences in boiling points. There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Evaporation removes a liquid from a solution to leave a solid. Distillation is the use of heat to separate. Difference Between Filtration Distillation And Chromatography.
From www.researchgate.net
Distillation for Water Purification Download Scientific Diagram Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. Chromatography involves solvent separation on a solid medium. The method chosen depends upon the type of mixture. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: While they share the common goal of separation, chromatography and distillation differ in their principles,. Difference Between Filtration Distillation And Chromatography.
From pediaa.com
Difference Between Fractional Distillation and Simple Distillation Process, Apparatus, Uses Difference Between Filtration Distillation And Chromatography Chromatography involves solvent separation on a solid medium. Chromatography involves solvent separation on a solid medium. There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation takes advantage of differences in boiling points. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: Distillation is the use of. Difference Between Filtration Distillation And Chromatography.
From www.slideserve.com
PPT Distillation & Chromatography PowerPoint Presentation, free download ID1964738 Difference Between Filtration Distillation And Chromatography Chromatography involves solvent separation on a solid medium. Evaporation removes a liquid from a solution to leave a solid. The vapor contains a larger proportion of the. Distillation takes advantage of differences in boiling points. Distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate components based on their. Chromatography involves solvent. Difference Between Filtration Distillation And Chromatography.
From www.pinterest.com
AQA GCSE Chemistry C1 Atoms, Elements and Compounds Mixtures Revision Notes,Video Question Ans Difference Between Filtration Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid. Chromatography involves solvent separation on a solid medium. The method chosen depends upon the type of mixture. The vapor contains a larger proportion of the. In general, the composition of the vapor above a liquid mixture differs from that of the liquid: Distillation is the use of heat to. Difference Between Filtration Distillation And Chromatography.