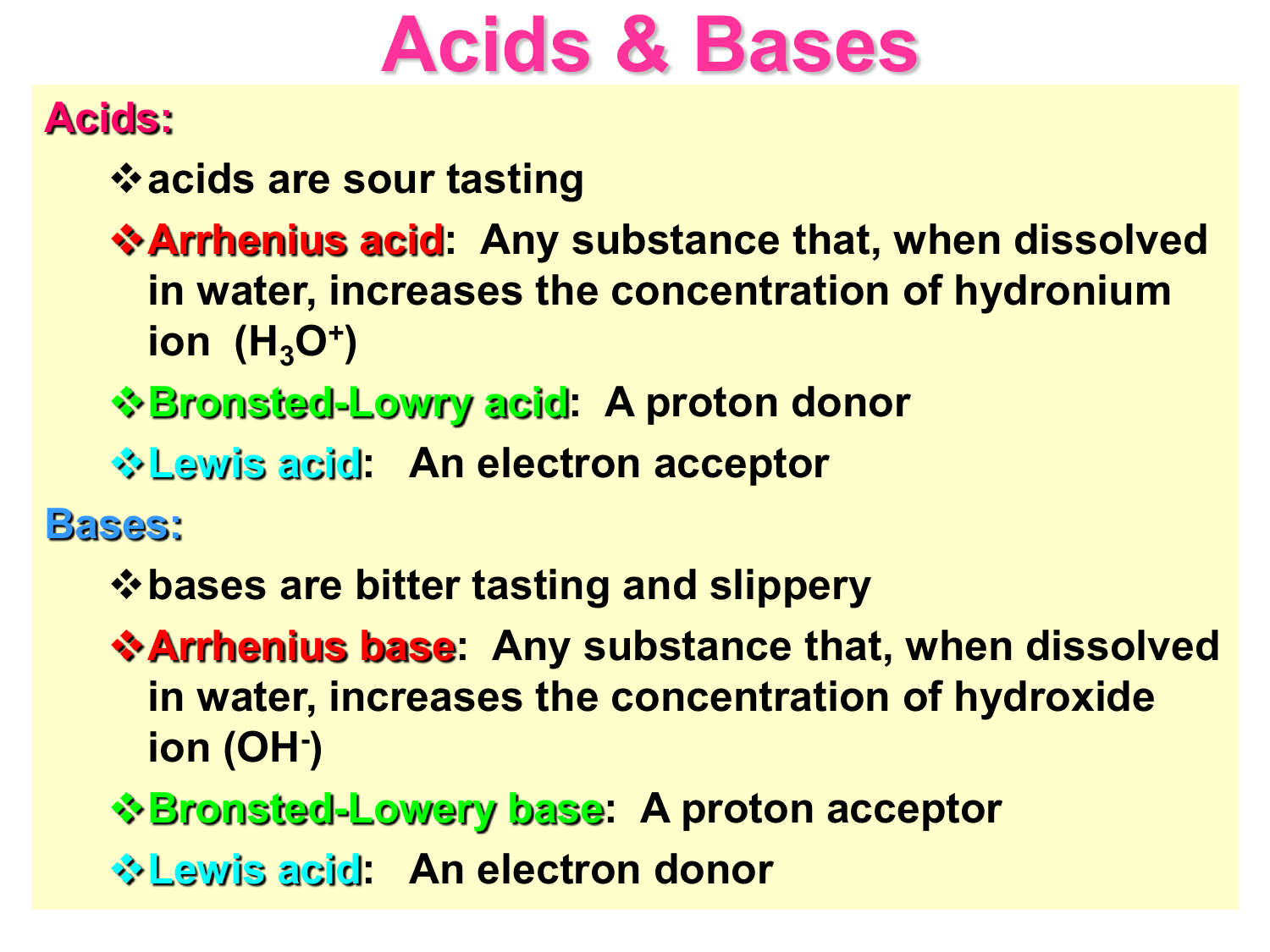
What Is Ha Acid Base . The equation takes its name for. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. This equilibrium constant is a. Imagine a generic acid, ha. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. It can be used to determine ph via titration. Updated on may 25, 2019.
from studylib.net
Imagine a generic acid, ha. This equilibrium constant is a. The equation takes its name for. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. It can be used to determine ph via titration. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Updated on may 25, 2019.
AcidBase Chemistry
What Is Ha Acid Base The equation takes its name for. This equilibrium constant is a. Imagine a generic acid, ha. Updated on may 25, 2019. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The equation takes its name for. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. It can be used to determine ph via titration.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID What Is Ha Acid Base This equilibrium constant is a. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Imagine a generic acid, ha. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The acid dissociation constant is the equilibrium. What Is Ha Acid Base.
From scienceandsf.com
Acids, Bases and pH. Scienceandsf A Blog Published by Robert A. Lawler What Is Ha Acid Base The equation takes its name for. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. Imagine a generic acid, ha. The acid dissociation constant is the. What Is Ha Acid Base.
From sciencenotes.org
Lewis Acid and Base Theory What Is Ha Acid Base The equation takes its name for. It can be used to determine ph via titration. This equilibrium constant is a. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. Imagine a generic acid, ha. Updated on may 25, 2019. The henderson hasselbalch equation finds the ph of a. What Is Ha Acid Base.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID What Is Ha Acid Base The equation takes its name for. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The acid dissociation constant is the equilibrium constant of the dissociation. What Is Ha Acid Base.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses What Is Ha Acid Base This equilibrium constant is a. Updated on may 25, 2019. The equation takes its name for. It can be used to determine ph via titration. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. Imagine a generic acid, ha. The conjugate base of a strong acid is a. What Is Ha Acid Base.
From www.alchem.ie
Equilibrium & Acid Base by Sarah Wegwerth What Is Ha Acid Base Imagine a generic acid, ha. Updated on may 25, 2019. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. This equilibrium constant is a. It can be used to determine ph via titration. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by. What Is Ha Acid Base.
From ecampusontario.pressbooks.pub
5.1 AcidBase Definitions & Conjugate AcidBase Pairs General What Is Ha Acid Base The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. This equilibrium constant is a. It can be used to determine ph via titration. Imagine a generic acid, ha. Updated on may 25, 2019. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a. What Is Ha Acid Base.
From emedia.rmit.edu.au
Acids, bases and salts Learning Lab What Is Ha Acid Base Updated on may 25, 2019. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. It can be used to determine ph via titration. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. The acid dissociation. What Is Ha Acid Base.
From www.worksheetsplanet.com
Acid and Bases Differences What Is Ha Acid Base This equilibrium constant is a. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. Updated on may 25, 2019. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. The acid dissociation constant is the equilibrium. What Is Ha Acid Base.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases What Is Ha Acid Base Imagine a generic acid, ha. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The acid dissociation constant is the equilibrium constant of the dissociation reaction. What Is Ha Acid Base.
From www.homeworkjoy.com
Main Differences Between Acid and Base What Is Ha Acid Base The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The equation takes its name for. It can be used to determine ph. What Is Ha Acid Base.
From www.sliderbase.com
Properties of Acids Bases Presentation Chemistry What Is Ha Acid Base It can be used to determine ph via titration. Updated on may 25, 2019. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak. What Is Ha Acid Base.
From www.chemicals.co.uk
What Is a Base in Chemistry? The Chemistry Blog What Is Ha Acid Base Updated on may 25, 2019. It can be used to determine ph via titration. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. Imagine a generic acid, ha. This equilibrium constant is a. The henderson hasselbalch equation finds the ph of a weak acid or poh of a. What Is Ha Acid Base.
From www.flinnsci.ca
AcidBase Strength Chart What Is Ha Acid Base This equilibrium constant is a. Imagine a generic acid, ha. It can be used to determine ph via titration. Updated on may 25, 2019. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The henderson hasselbalch equation finds the ph of a weak. What Is Ha Acid Base.
From ecampusontario.pressbooks.pub
5.1 AcidBase Definitions & Conjugate AcidBase Pairs General What Is Ha Acid Base Imagine a generic acid, ha. This equilibrium constant is a. It can be used to determine ph via titration. Updated on may 25, 2019. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The equation takes its name for. The henderson hasselbalch equation. What Is Ha Acid Base.
From sciencenotes.org
AcidBase Chemistry What Is Ha Acid Base It can be used to determine ph via titration. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. This equilibrium constant is a. Imagine a generic. What Is Ha Acid Base.
From scienceinfo.com
AcidBase Reaction (Neutralization Reaction) What Is Ha Acid Base The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. The equation takes its name for. Imagine a generic acid, ha. It can be used to determine ph via titration. Updated on may 25, 2019. The henderson hasselbalch equation finds the ph of a weak acid or poh of. What Is Ha Acid Base.
From www.teachoo.com
10+ Acids used in Daily Life (with images) Teachoo Science What Is Ha Acid Base It can be used to determine ph via titration. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. Updated on may 25, 2019. Imagine a generic acid, ha. This equilibrium constant is a. The equation takes its name for. The conjugate base of a strong acid is a. What Is Ha Acid Base.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Is Ha Acid Base Imagine a generic acid, ha. This equilibrium constant is a. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. The equation takes its name for. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. The conjugate base of a strong. What Is Ha Acid Base.
From pediaa.com
Difference Between Acid and Base What Is Ha Acid Base It can be used to determine ph via titration. The equation takes its name for. Imagine a generic acid, ha. This equilibrium constant is a. Updated on may 25, 2019. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The acid dissociation constant. What Is Ha Acid Base.
From www.osmosis.org
Overview of AcidBase Regulation Osmosis Video Library What Is Ha Acid Base Updated on may 25, 2019. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. The acid dissociation constant is the equilibrium constant of the dissociation reaction. What Is Ha Acid Base.
From www.sssi.in
Acids, Bases, and Salts Key Concepts and Properties Explained What Is Ha Acid Base This equilibrium constant is a. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. Imagine a generic acid, ha. The equation takes its name for. Updated on may 25, 2019. The henderson hasselbalch equation finds the ph of a weak acid or poh. What Is Ha Acid Base.
From www.straighthealthcare.com
Normal acidbase physiology illustration What Is Ha Acid Base The equation takes its name for. It can be used to determine ph via titration. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Imagine a. What Is Ha Acid Base.
From www.teachoo.com
Acids and it's Properties Definition [with Flowchart and Examples] What Is Ha Acid Base Updated on may 25, 2019. Imagine a generic acid, ha. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. The equation takes its name for. It can be used to determine ph via titration. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of. What Is Ha Acid Base.
From kidspressmagazine.com
Acids and Bases What Is Ha Acid Base The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. It can be used to determine ph via titration. This equilibrium constant is a. Imagine a generic acid, ha. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Updated on may. What Is Ha Acid Base.
From examples.yourdictionary.com
Difference Between Acids and Bases Key Properties What Is Ha Acid Base The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. This equilibrium constant is a. It can be used to determine ph via titration. The conjugate base of a strong acid is. What Is Ha Acid Base.
From sciencenotes.org
Facts About Acids and Bases What Is Ha Acid Base It can be used to determine ph via titration. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. Updated on may 25, 2019. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak. What Is Ha Acid Base.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs What Is Ha Acid Base It can be used to determine ph via titration. This equilibrium constant is a. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k. What Is Ha Acid Base.
From studylib.net
AcidBase Chemistry What Is Ha Acid Base Imagine a generic acid, ha. This equilibrium constant is a. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. The henderson hasselbalch. What Is Ha Acid Base.
From dashamlav.com
Acid vs Base Table of Differences between Acid and Base What Is Ha Acid Base Updated on may 25, 2019. The equation takes its name for. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. Imagine a generic acid, ha. This equilibrium constant is a. It can be used to determine ph via titration. The acid dissociation constant. What Is Ha Acid Base.
From www.youtube.com
Identifying Acids, Bases, & Conjugate Acids & Bases in an Acid/Base What Is Ha Acid Base This equilibrium constant is a. Updated on may 25, 2019. The equation takes its name for. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. Imagine a generic acid, ha. It can be used to determine ph via titration. The henderson hasselbalch equation. What Is Ha Acid Base.
From www.teachoo.com
Difference between Acid and Base (in Table form) Teachoo What Is Ha Acid Base Imagine a generic acid, ha. Updated on may 25, 2019. It can be used to determine ph via titration. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The henderson hasselbalch equation finds the ph of a weak acid or poh of a. What Is Ha Acid Base.
From www.slideserve.com
PPT Acid Base Chemistry An acid is a H + (proton) donor . General What Is Ha Acid Base Imagine a generic acid, ha. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. It can be used to determine ph via titration. The acid dissociation. What Is Ha Acid Base.
From morioh.com
Acids and Bases Basic Introduction Chemistry What Is Ha Acid Base Imagine a generic acid, ha. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. It can be used to determine ph via titration. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. Updated on may 25, 2019. The conjugate base. What Is Ha Acid Base.
From mavink.com
Acid Base Map What Is Ha Acid Base The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. The equation takes its name for. This equilibrium constant is a. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. Imagine a. What Is Ha Acid Base.