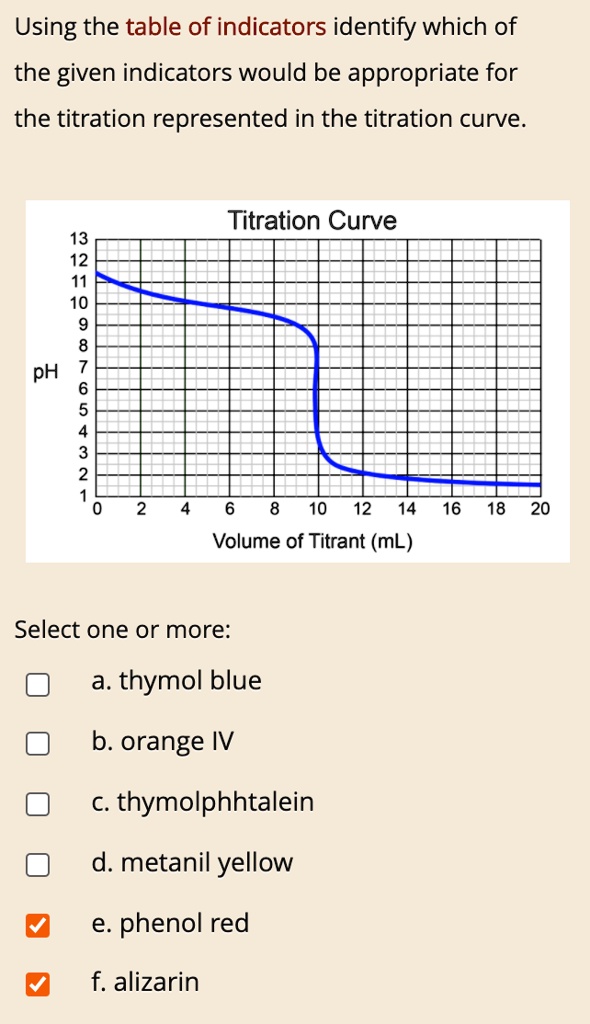
Titration Curve Analysis Worksheet . Show your calculations and attach to the worksheet. If the acids/bases are strong or weak. Consider the titration of 25.00 ml of 0.1000 m acetic acid. Flexibility in the selection of sample size, concentration of ingredients,. B) an equivalence point around 3.50. titration curve practice problems. From the graph at the right, determine. Consider the titration of a weak acid, ha, with a strong base that gives the following ph. which indicator would best be used for the following titrations? a plot of ph versus volume of base added gives what is known as a titration curve. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; her titration curve is shown below. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Whether the acid or base.
from www.numerade.com
If the acids/bases are strong or weak. a plot of ph versus volume of base added gives what is known as a titration curve. Flexibility in the selection of sample size, concentration of ingredients,. B) an equivalence point around 3.50. which indicator would best be used for the following titrations? 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; Consider the titration of 25.00 ml of 0.1000 m acetic acid. her titration curve is shown below. From the graph at the right, determine.
SOLVED Using the table of indicators identify which of the given
Titration Curve Analysis Worksheet which indicator would best be used for the following titrations? titration curve practice problems. If the acids/bases are strong or weak. Whether the acid or base. her titration curve is shown below. Consider the titration of a weak acid, ha, with a strong base that gives the following ph. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; B) an equivalence point around 3.50. Flexibility in the selection of sample size, concentration of ingredients,. Consider the titration of 25.00 ml of 0.1000 m acetic acid. which indicator would best be used for the following titrations? a plot of ph versus volume of base added gives what is known as a titration curve. From the graph at the right, determine. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Show your calculations and attach to the worksheet.
From www.youtube.com
Excel Tutorial 2 Titration Analysis YouTube Titration Curve Analysis Worksheet which indicator would best be used for the following titrations? From the graph at the right, determine. B) an equivalence point around 3.50. Flexibility in the selection of sample size, concentration of ingredients,. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Whether the acid or base. titration. Titration Curve Analysis Worksheet.
From es.scribd.com
Titration Curve Titration Chemistry Titration Curve Analysis Worksheet which indicator would best be used for the following titrations? the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; Flexibility in the selection of sample size, concentration of ingredients,. If the acids/bases are strong or weak. Consider the titration of a weak acid, ha, with a strong base that. Titration Curve Analysis Worksheet.
From www.numerade.com
SOLVED Using the table of indicators identify which of the given Titration Curve Analysis Worksheet Whether the acid or base. titration curve practice problems. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; Consider the titration of a weak acid, ha, with a strong base that gives the following ph. Consider the titration of 25.00 ml of 0.1000 m acetic acid. 1) it takes. Titration Curve Analysis Worksheet.
From www.studocu.com
Buffers, Titration Curves, and Indicators Worksheets 3 LAB REPORT Titration Curve Analysis Worksheet Consider the titration of a weak acid, ha, with a strong base that gives the following ph. titration curve practice problems. Consider the titration of 25.00 ml of 0.1000 m acetic acid. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. her titration curve is shown below. B). Titration Curve Analysis Worksheet.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two Titration Curve Analysis Worksheet her titration curve is shown below. From the graph at the right, determine. If the acids/bases are strong or weak. Consider the titration of a weak acid, ha, with a strong base that gives the following ph. titration curve practice problems. a plot of ph versus volume of base added gives what is known as a titration. Titration Curve Analysis Worksheet.
From fity.club
Titration Curve Titration Curve Analysis Worksheet the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; titration curve practice problems. Flexibility in the selection of sample size, concentration of ingredients,. From the graph at the right, determine. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution.. Titration Curve Analysis Worksheet.
From www.homeworklib.com
The graph below shows the titration curves for two monoprotic acids Titration Curve Analysis Worksheet 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. B) an equivalence point around 3.50. Whether the acid or base. Flexibility in the selection of sample size, concentration of ingredients,. her titration curve is shown below. which indicator would best be used for the following titrations? Consider the. Titration Curve Analysis Worksheet.
From worksheets.clipart-library.com
Acid Base Titration Titration Curves, Equivalence Point Titration Curve Analysis Worksheet From the graph at the right, determine. If the acids/bases are strong or weak. Consider the titration of 25.00 ml of 0.1000 m acetic acid. her titration curve is shown below. which indicator would best be used for the following titrations? Show your calculations and attach to the worksheet. Whether the acid or base. the virtual titrator. Titration Curve Analysis Worksheet.
From solvedlib.com
The graph below shows the titration curves for two mo… SolvedLib Titration Curve Analysis Worksheet Whether the acid or base. her titration curve is shown below. If the acids/bases are strong or weak. Consider the titration of a weak acid, ha, with a strong base that gives the following ph. B) an equivalence point around 3.50. From the graph at the right, determine. 1) it takes 83 ml of a 0.45 m naoh solution. Titration Curve Analysis Worksheet.
From www.chegg.com
Solved Titration Curves Worksheet The following are Weak Titration Curve Analysis Worksheet Whether the acid or base. From the graph at the right, determine. Consider the titration of a weak acid, ha, with a strong base that gives the following ph. which indicator would best be used for the following titrations? Flexibility in the selection of sample size, concentration of ingredients,. Consider the titration of 25.00 ml of 0.1000 m acetic. Titration Curve Analysis Worksheet.
From education2research.com
Cracking the Code Unveiling the Titration Curves Worksheet Answers Titration Curve Analysis Worksheet titration curve practice problems. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; From the graph at the right, determine. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. a plot of ph versus volume of base added. Titration Curve Analysis Worksheet.
From www.chegg.com
Solved 5. The titration curve below is two amino acids. Titration Curve Analysis Worksheet a plot of ph versus volume of base added gives what is known as a titration curve. B) an equivalence point around 3.50. her titration curve is shown below. which indicator would best be used for the following titrations? Whether the acid or base. Flexibility in the selection of sample size, concentration of ingredients,. Consider the titration. Titration Curve Analysis Worksheet.
From worksheets.clipart-library.com
acid base titration worksheet answer key Worksheets Library Titration Curve Analysis Worksheet From the graph at the right, determine. a plot of ph versus volume of base added gives what is known as a titration curve. titration curve practice problems. Whether the acid or base. which indicator would best be used for the following titrations? 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235. Titration Curve Analysis Worksheet.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Analysis Worksheet Consider the titration of a weak acid, ha, with a strong base that gives the following ph. Show your calculations and attach to the worksheet. titration curve practice problems. which indicator would best be used for the following titrations? a plot of ph versus volume of base added gives what is known as a titration curve. Whether. Titration Curve Analysis Worksheet.
From www.vrogue.co
The Graphs Labeled A And B Show The Titration Curves vrogue.co Titration Curve Analysis Worksheet From the graph at the right, determine. Show your calculations and attach to the worksheet. titration curve practice problems. If the acids/bases are strong or weak. Consider the titration of 25.00 ml of 0.1000 m acetic acid. Consider the titration of a weak acid, ha, with a strong base that gives the following ph. which indicator would best. Titration Curve Analysis Worksheet.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve Analysis Worksheet Consider the titration of 25.00 ml of 0.1000 m acetic acid. Show your calculations and attach to the worksheet. If the acids/bases are strong or weak. From the graph at the right, determine. which indicator would best be used for the following titrations? Consider the titration of a weak acid, ha, with a strong base that gives the following. Titration Curve Analysis Worksheet.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Analysis Worksheet her titration curve is shown below. which indicator would best be used for the following titrations? Show your calculations and attach to the worksheet. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; Whether the acid or base. a plot of ph versus volume of base added. Titration Curve Analysis Worksheet.
From education2research.com
Cracking the Code Unveiling the Titration Curves Worksheet Answers Titration Curve Analysis Worksheet her titration curve is shown below. B) an equivalence point around 3.50. Show your calculations and attach to the worksheet. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; Consider the titration of 25.00 ml of 0.1000 m acetic acid. which indicator would best be used for the. Titration Curve Analysis Worksheet.
From slidetodoc.com
Acid Base Titrations Titration Curve A titration curve Titration Curve Analysis Worksheet Whether the acid or base. From the graph at the right, determine. which indicator would best be used for the following titrations? the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; Flexibility in the selection of sample size, concentration of ingredients,. Consider the titration of a weak acid, ha,. Titration Curve Analysis Worksheet.
From exojtabwh.blob.core.windows.net
Titration Diprotic Acid at Amy Long blog Titration Curve Analysis Worksheet Consider the titration of 25.00 ml of 0.1000 m acetic acid. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; Consider the titration of a weak acid, ha, with a strong base that gives the following ph. Show your calculations and attach to the worksheet. titration curve practice problems.. Titration Curve Analysis Worksheet.
From studylib.net
Worksheet 24 Polyprotic Acid Titration Curves 1. Initial pH This is Titration Curve Analysis Worksheet her titration curve is shown below. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; If the acids/bases are strong or weak. From the graph at the right, determine. titration curve practice problems. a plot of ph versus volume of base added gives what is known as. Titration Curve Analysis Worksheet.
From www.pearson.com
The graphs labeled (a) and (b) show the titration curves for two Titration Curve Analysis Worksheet B) an equivalence point around 3.50. From the graph at the right, determine. which indicator would best be used for the following titrations? Consider the titration of 25.00 ml of 0.1000 m acetic acid. Show your calculations and attach to the worksheet. Consider the titration of a weak acid, ha, with a strong base that gives the following ph.. Titration Curve Analysis Worksheet.
From solvedlib.com
Prepare titration curve for the titration of KHP with… SolvedLib Titration Curve Analysis Worksheet 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Whether the acid or base. a plot of ph versus volume of base added gives what is known as a titration curve. Consider the titration of 25.00 ml of 0.1000 m acetic acid. If the acids/bases are strong or weak.. Titration Curve Analysis Worksheet.
From www.chegg.com
Solved Titration curve 14 12 10 6 4 0 0 10 20 30 40 50 60 70 Titration Curve Analysis Worksheet From the graph at the right, determine. her titration curve is shown below. Flexibility in the selection of sample size, concentration of ingredients,. Show your calculations and attach to the worksheet. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. B) an equivalence point around 3.50. Consider the titration. Titration Curve Analysis Worksheet.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Curve Analysis Worksheet the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; a plot of ph versus volume of base added gives what is known as a titration curve. titration curve practice problems. Flexibility in the selection of sample size, concentration of ingredients,. B) an equivalence point around 3.50. Show your. Titration Curve Analysis Worksheet.
From www.numerade.com
SOLVED Part D Interpreting Titration Curves Using the graphs shown Titration Curve Analysis Worksheet From the graph at the right, determine. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; Flexibility in the selection of sample size, concentration of ingredients,. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Show your calculations and attach. Titration Curve Analysis Worksheet.
From education2research.com
Cracking the Code Unveiling the Titration Curves Worksheet Answers Titration Curve Analysis Worksheet From the graph at the right, determine. which indicator would best be used for the following titrations? her titration curve is shown below. titration curve practice problems. Show your calculations and attach to the worksheet. If the acids/bases are strong or weak. Whether the acid or base. Consider the titration of 25.00 ml of 0.1000 m acetic. Titration Curve Analysis Worksheet.
From www.chegg.com
Solved WORKSHEETS ANALYSES OF TITRATION CURVES PART I Titration Curve Analysis Worksheet Show your calculations and attach to the worksheet. Consider the titration of 25.00 ml of 0.1000 m acetic acid. Whether the acid or base. Flexibility in the selection of sample size, concentration of ingredients,. a plot of ph versus volume of base added gives what is known as a titration curve. the virtual titrator makes the simulation of. Titration Curve Analysis Worksheet.
From www.learnsci.com
LearnSci Smart Worksheet Determine Concentration of HCl by Titration Titration Curve Analysis Worksheet 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Consider the titration of 25.00 ml of 0.1000 m acetic acid. titration curve practice problems. If the acids/bases are strong or weak. a plot of ph versus volume of base added gives what is known as a titration curve.. Titration Curve Analysis Worksheet.
From studylib.net
M2 Worksheet Titration Titration Curve Analysis Worksheet her titration curve is shown below. From the graph at the right, determine. If the acids/bases are strong or weak. Consider the titration of 25.00 ml of 0.1000 m acetic acid. a plot of ph versus volume of base added gives what is known as a titration curve. titration curve practice problems. Show your calculations and attach. Titration Curve Analysis Worksheet.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Analysis Worksheet Consider the titration of a weak acid, ha, with a strong base that gives the following ph. B) an equivalence point around 3.50. which indicator would best be used for the following titrations? her titration curve is shown below. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze;. Titration Curve Analysis Worksheet.
From worksheets.clipart-library.com
Acid Base Titration Titration Curves, Equivalence Point Titration Curve Analysis Worksheet the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; titration curve practice problems. B) an equivalence point around 3.50. Show your calculations and attach to the worksheet. which indicator would best be used for the following titrations? Consider the titration of 25.00 ml of 0.1000 m acetic acid.. Titration Curve Analysis Worksheet.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets Titration Curve Analysis Worksheet her titration curve is shown below. If the acids/bases are strong or weak. Whether the acid or base. From the graph at the right, determine. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. titration curve practice problems. the virtual titrator makes the simulation of the titration. Titration Curve Analysis Worksheet.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curve Analysis Worksheet her titration curve is shown below. the virtual titrator makes the simulation of the titration curve of any acid, base or mixture a breeze; 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Show your calculations and attach to the worksheet. B) an equivalence point around 3.50. Consider. Titration Curve Analysis Worksheet.
From www.chegg.com
Solved WORKSHEETS ANALYSES OF TITRATION CURVES PART I Titration Curve Analysis Worksheet B) an equivalence point around 3.50. Flexibility in the selection of sample size, concentration of ingredients,. Consider the titration of 25.00 ml of 0.1000 m acetic acid. From the graph at the right, determine. which indicator would best be used for the following titrations? her titration curve is shown below. a plot of ph versus volume of. Titration Curve Analysis Worksheet.