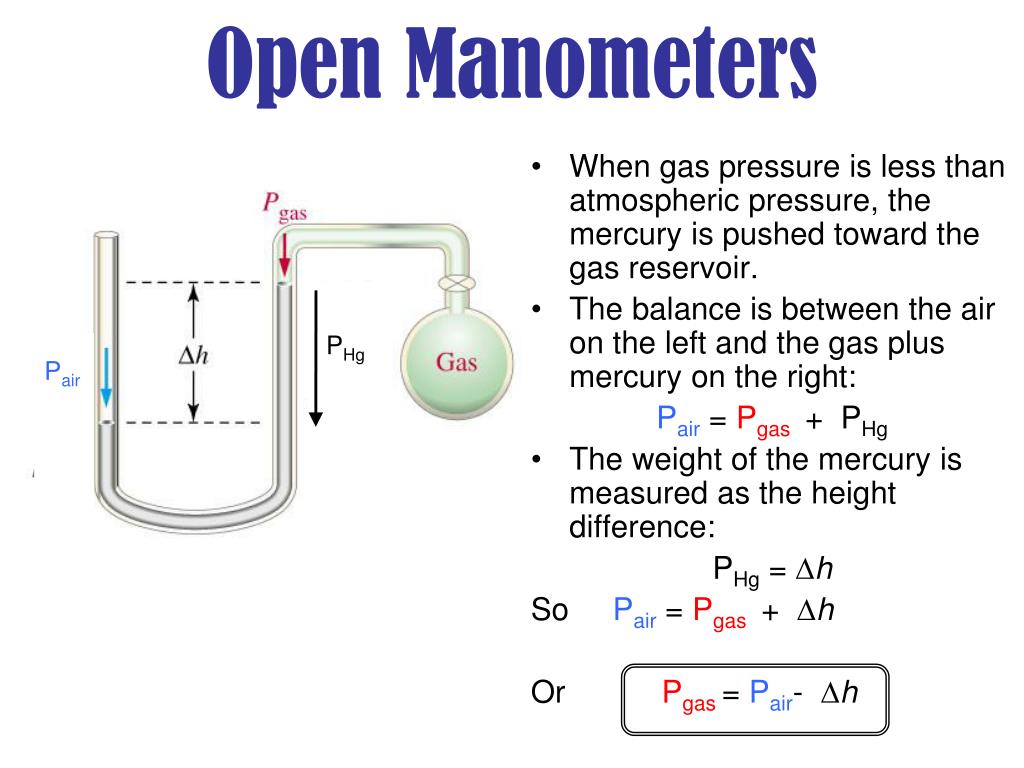
Mercury Manometer Formula . Carefully read the heights of the two columns of mercury in the. 760 mm hg = 1 atmosphere). Small pressure differences, such as those experienced in experimental wind. A mercury barometer measures atmospheric pressure. A mercury manometer is used to measure the pressure of a gas in a flask. The pressure due to the mercury’s weight, h\(\rho\)g, equals atmospheric pressure. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. Large pressure differences are measured with heavy fluids, such as mercury (e.g. The mercury manometer is the most common type, which uses mercury as. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. Use a manometer to measure pressure. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. An illustration of a manometer connected to a system.
from www.slideserve.com
The pressure due to the mercury’s weight, h\(\rho\)g, equals atmospheric pressure. 760 mm hg = 1 atmosphere). The mercury manometer is the most common type, which uses mercury as. A mercury manometer is used to measure the pressure of a gas in a flask. Small pressure differences, such as those experienced in experimental wind. An illustration of a manometer connected to a system. Large pressure differences are measured with heavy fluids, such as mercury (e.g. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. A mercury barometer measures atmospheric pressure. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to.
PPT Measuring Pressure PowerPoint Presentation, free download ID
Mercury Manometer Formula 760 mm hg = 1 atmosphere). An illustration of a manometer connected to a system. 760 mm hg = 1 atmosphere). The mercury manometer is the most common type, which uses mercury as. The pressure due to the mercury’s weight, h\(\rho\)g, equals atmospheric pressure. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. Large pressure differences are measured with heavy fluids, such as mercury (e.g. Use a manometer to measure pressure. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. Small pressure differences, such as those experienced in experimental wind. A mercury manometer is used to measure the pressure of a gas in a flask. A mercury barometer measures atmospheric pressure. Carefully read the heights of the two columns of mercury in the.
From www.numerade.com
Mercury Manometer Pressure Units Schematic diagrams of three opentube Mercury Manometer Formula As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. Carefully read the heights of the two columns of mercury in the. The mercury manometer is the most common type, which uses mercury as. An illustration of a manometer connected to a system. Small pressure differences, such as. Mercury Manometer Formula.
From www.chemistrytutorials.org
Measuring Pressure of Gas and Manometers with Examples Online Mercury Manometer Formula An illustration of a manometer connected to a system. Large pressure differences are measured with heavy fluids, such as mercury (e.g. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg). Mercury Manometer Formula.
From www.youtube.com
Measuring Absolute and Gauge Pressure of Fluids Using U Tube Manometers Mercury Manometer Formula A mercury barometer measures atmospheric pressure. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. Carefully read the heights of the two columns of mercury in the. A mercury manometer is used to measure the pressure of a gas in a flask. An illustration of a manometer. Mercury Manometer Formula.
From www.solutionspile.com
[Solved] Consider the image of a mercury manometer below. Mercury Manometer Formula Small pressure differences, such as those experienced in experimental wind. The mercury manometer is the most common type, which uses mercury as. 760 mm hg = 1 atmosphere). The pressure due to the mercury’s weight, h\(\rho\)g, equals atmospheric pressure. An illustration of a manometer connected to a system. Use a manometer to measure pressure. A mercury manometer is used to. Mercury Manometer Formula.
From www.doubtnut.com
An open ended mercury manometer is used to measure the pressure ex Mercury Manometer Formula A mercury manometer is used to measure the pressure of a gas in a flask. Carefully read the heights of the two columns of mercury in the. 760 mm hg = 1 atmosphere). As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. Large pressure differences are measured. Mercury Manometer Formula.
From www.numerade.com
SOLVEDA mercury manometer (ρ=13,600 kg / m^3) is connected to an air Mercury Manometer Formula Use a manometer to measure pressure. An illustration of a manometer connected to a system. The mercury manometer is the most common type, which uses mercury as. Large pressure differences are measured with heavy fluids, such as mercury (e.g. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system.. Mercury Manometer Formula.
From www.chegg.com
Solved A mercury manometer is used to measure the pressure Mercury Manometer Formula The mercury manometer is the most common type, which uses mercury as. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask,. Mercury Manometer Formula.
From mercury-drugs.blogspot.com
Mercury U Tube Manometer Calculations Mercury Drugs Mercury Manometer Formula Carefully read the heights of the two columns of mercury in the. Large pressure differences are measured with heavy fluids, such as mercury (e.g. The mercury manometer is the most common type, which uses mercury as. Use a manometer to measure pressure. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or. Mercury Manometer Formula.
From www.slideserve.com
PPT Pressure Measurements PowerPoint Presentation, free download ID Mercury Manometer Formula Small pressure differences, such as those experienced in experimental wind. The mercury manometer is the most common type, which uses mercury as. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or. Mercury Manometer Formula.
From www.slideserve.com
PPT Measuring Pressure PowerPoint Presentation, free download ID Mercury Manometer Formula In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. Use a manometer to measure pressure. A mercury barometer measures atmospheric pressure. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. Small pressure differences, such as. Mercury Manometer Formula.
From www.youtube.com
Manometer Example YouTube Mercury Manometer Formula The pressure due to the mercury’s weight, h\(\rho\)g, equals atmospheric pressure. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. A mercury barometer measures atmospheric pressure. The mercury manometer is the most common type, which uses mercury as. Small pressure differences, such as those experienced in experimental wind.. Mercury Manometer Formula.
From www.slideserve.com
PPT Chapter 11 Properties of Gases PowerPoint Presentation, free Mercury Manometer Formula If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. Large pressure differences are measured with heavy fluids, such as mercury (e.g. The mercury manometer is the most common type, which uses mercury as. A mercury barometer measures atmospheric pressure. A mercury. Mercury Manometer Formula.
From www.chegg.com
Solved Question A8 Gas A Utube manometer used to measure Mercury Manometer Formula The mercury manometer is the most common type, which uses mercury as. Use a manometer to measure pressure. An illustration of a manometer connected to a system. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. Large pressure differences are measured with heavy fluids, such as mercury (e.g.. Mercury Manometer Formula.
From www.chegg.com
Solved The mercury manometer shown here is used to measure Mercury Manometer Formula An illustration of a manometer connected to a system. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. Small pressure differences, such as those experienced in experimental wind. A mercury barometer measures atmospheric pressure. Carefully read the heights of the two columns of mercury in the. A mercury. Mercury Manometer Formula.
From www.coursehero.com
[Solved] As shown in the figure below, an inclined manometer is used to Mercury Manometer Formula If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. Carefully read the heights of the two columns of mercury in the. Small pressure differences, such as those experienced in experimental wind. A mercury manometer is used to measure the pressure of. Mercury Manometer Formula.
From www.scribd.com
Mercury Manometer PDF Pressure Pressure Measurement Mercury Manometer Formula The pressure due to the mercury’s weight, h\(\rho\)g, equals atmospheric pressure. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. 760 mm hg = 1 atmosphere). Small pressure differences, such as those experienced in experimental wind. Large pressure differences are measured with heavy fluids, such as mercury (e.g.. Mercury Manometer Formula.
From slidetodoc.com
FUNDAMENTALS OF FLUID MECHANICS Chapter 2 Fluids at Mercury Manometer Formula Small pressure differences, such as those experienced in experimental wind. A mercury manometer is used to measure the pressure of a gas in a flask. An illustration of a manometer connected to a system. Large pressure differences are measured with heavy fluids, such as mercury (e.g. 760 mm hg = 1 atmosphere). Use a manometer to measure pressure. In this. Mercury Manometer Formula.
From spmphysics.onlinetuition.com.my
Manometer SPM Physics Form 4/Form 5 Revision Notes Mercury Manometer Formula If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. Large pressure differences are measured with heavy fluids, such as mercury (e.g. An illustration of a manometer connected to a system. The mercury manometer is the most common type, which uses mercury. Mercury Manometer Formula.
From www.numerade.com
SOLVED The mercury manometer, shown in figure Q3, is used to measure Mercury Manometer Formula Small pressure differences, such as those experienced in experimental wind. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. A mercury barometer measures atmospheric pressure. An illustration of a manometer connected to a system. Carefully read the heights of the two. Mercury Manometer Formula.
From tutorbin.com
Solved A mercury manometer (p = 13,600 kg/m^3) is connected to an air Mercury Manometer Formula Small pressure differences, such as those experienced in experimental wind. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. If mercury is the liquid in the. Mercury Manometer Formula.
From www.chegg.com
Solved A manometer which has mercury in it, as shown below, Mercury Manometer Formula In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. The mercury manometer is the most common type, which uses mercury as. Use a manometer to measure. Mercury Manometer Formula.
From www.coursehero.com
[Solved] . 1) A mercury manometer is connected to a large reservoir of Mercury Manometer Formula A mercury barometer measures atmospheric pressure. An illustration of a manometer connected to a system. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer. Mercury Manometer Formula.
From www.toppr.com
An open ended mercury manometer is used to manometer is used to measure Mercury Manometer Formula The mercury manometer is the most common type, which uses mercury as. A mercury manometer is used to measure the pressure of a gas in a flask. Large pressure differences are measured with heavy fluids, such as mercury (e.g. A mercury barometer measures atmospheric pressure. Small pressure differences, such as those experienced in experimental wind. 760 mm hg = 1. Mercury Manometer Formula.
From www.nandantechnicals.com
UTube Manometer Mercury Manometer Formula 760 mm hg = 1 atmosphere). Use a manometer to measure pressure. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. Carefully read the heights of the two columns of mercury in the. Small pressure differences, such as those experienced in experimental wind. A mercury manometer is used. Mercury Manometer Formula.
From byjus.com
A manometer consists of a U shaped tube containing water. One side is Mercury Manometer Formula An illustration of a manometer connected to a system. A mercury manometer is used to measure the pressure of a gas in a flask. 760 mm hg = 1 atmosphere). Large pressure differences are measured with heavy fluids, such as mercury (e.g. The mercury manometer is the most common type, which uses mercury as. Small pressure differences, such as those. Mercury Manometer Formula.
From mercury-drugs.blogspot.com
Mercury U Tube Manometer Calculations Mercury Drugs Mercury Manometer Formula The mercury manometer is the most common type, which uses mercury as. A mercury manometer is used to measure the pressure of a gas in a flask. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. Small pressure differences, such as. Mercury Manometer Formula.
From www.chegg.com
Solved 1 of 5 The diagram shows a mercury manometer used to Mercury Manometer Formula The mercury manometer is the most common type, which uses mercury as. The pressure due to the mercury’s weight, h\(\rho\)g, equals atmospheric pressure. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. A mercury manometer is used to measure the pressure of a gas in a flask.. Mercury Manometer Formula.
From engineerexcel.com
Manometer Equation Calculate Pressure from a Manometer Reading Mercury Manometer Formula A mercury manometer is used to measure the pressure of a gas in a flask. Large pressure differences are measured with heavy fluids, such as mercury (e.g. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. Carefully read the heights of. Mercury Manometer Formula.
From www.chegg.com
Solved A mercury manometer is used to measure the pressure Mercury Manometer Formula Large pressure differences are measured with heavy fluids, such as mercury (e.g. Carefully read the heights of the two columns of mercury in the. 760 mm hg = 1 atmosphere). A mercury manometer is used to measure the pressure of a gas in a flask. The mercury manometer is the most common type, which uses mercury as. Use a manometer. Mercury Manometer Formula.
From www.coursehero.com
[Solved] A well type manometer uses mercury as the manometric fluid Mercury Manometer Formula The mercury manometer is the most common type, which uses mercury as. Carefully read the heights of the two columns of mercury in the. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. A mercury barometer measures atmospheric pressure. A mercury manometer is used to measure the. Mercury Manometer Formula.
From philschatz.com
Gauge Pressure, Absolute Pressure, and Pressure Measurement · Physics Mercury Manometer Formula If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. An illustration of a manometer connected to a system. 760 mm hg = 1 atmosphere). Carefully read the heights of the two columns of mercury in the. In this example, we have. Mercury Manometer Formula.
From chemistrytalk.org
Barometers and Manometers ChemTalk Mercury Manometer Formula The pressure due to the mercury’s weight, h\(\rho\)g, equals atmospheric pressure. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. Small pressure differences, such as those experienced in experimental wind. Carefully read the heights of the two columns of mercury in the. As shown in figure 2 b. Mercury Manometer Formula.
From www.chegg.com
Solved 3. A Utube manometer contains mercury of density Mercury Manometer Formula Carefully read the heights of the two columns of mercury in the. 760 mm hg = 1 atmosphere). Large pressure differences are measured with heavy fluids, such as mercury (e.g. Use a manometer to measure pressure. As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. An illustration. Mercury Manometer Formula.
From byjus.com
A manometer tube contains mercury of density 13.6 x 103 kg m3. What Mercury Manometer Formula In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. If mercury is the liquid in the manometer, the pressure is expressed in inches of mercury (inhg) or millimeters of mercury (mmhg) different manometer equations are used to. Carefully read the heights of the two columns of mercury in. Mercury Manometer Formula.
From www.toppr.com
An open ended mercury manometer is used to manometer is used to measure Mercury Manometer Formula As shown in figure 2 b , the level of mercury is higher in the arm connected to the flask, but the. Small pressure differences, such as those experienced in experimental wind. In this example, we have mercury (ρ₁=13.6 g/cm³ or 13,600 kg/m³) and water (ρ₂=1.0 g/cm³ or 1,000 kg/m³) in the system. An illustration of a manometer connected to. Mercury Manometer Formula.