Titration Curve Explanation . A titration curve is a graphical representation of the ph of a solution during a titration. Both equivalence points are visible. In the beginning, the solution has a All acid titration curves follow the same basic shapes. The equivalence point of a titration. How do you explain the shape of a titration curve? It provides valuable information about the reaction under study and helps understand the results obtained. The figure below shows two different. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Everything you need to know for a level [2,3,5] when interpreting a titration curve, it is essential to understand its key features. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. What are ph titration curves? Titration is a technique used in neutralisation reactions between acids and alkalis.
from slidetodoc.com
A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different. In the beginning, the solution has a A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. How do you explain the shape of a titration curve? Both equivalence points are visible. The equivalence point of a titration. Titration is a technique used in neutralisation reactions between acids and alkalis. [2,3,5] when interpreting a titration curve, it is essential to understand its key features. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve.
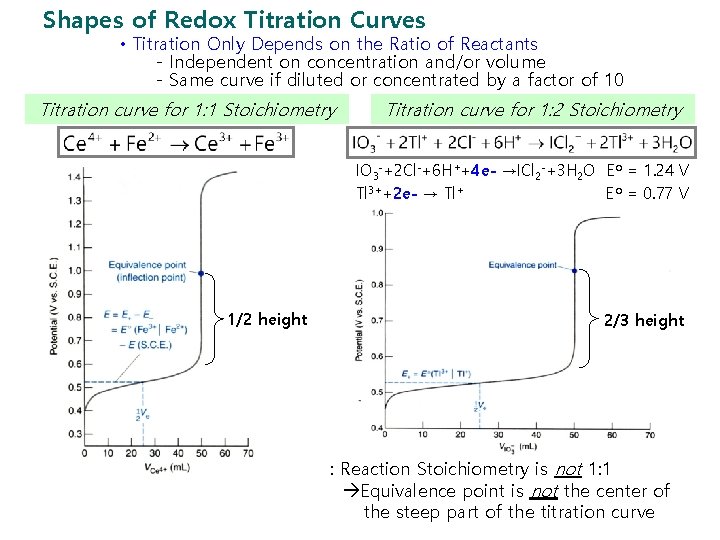
Chapter 15 Redox Titrations 15 1 The Shape
Titration Curve Explanation All acid titration curves follow the same basic shapes. A titration curve is a graphical representation of the ph of a solution during a titration. Everything you need to know for a level And why is the equivalence point not always at ph7? In the beginning, the solution has a [2,3,5] when interpreting a titration curve, it is essential to understand its key features. Titration is a technique used in neutralisation reactions between acids and alkalis. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. What are ph titration curves? The titration curve is a graphical representation of the ph or other property changes during a titration experiment. How do you explain the shape of a titration curve? The equivalence point of a titration. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. It provides valuable information about the reaction under study and helps understand the results obtained. All acid titration curves follow the same basic shapes. The figure below shows two different.
From quizlet.com
Explain what a titration curve is, and sketch its shape. Quizlet Titration Curve Explanation And why is the equivalence point not always at ph7? All acid titration curves follow the same basic shapes. Both equivalence points are visible. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. A typical titration curve of a diprotic acid,. Titration Curve Explanation.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Explanation The figure below shows two different. Both equivalence points are visible. How do you explain the shape of a titration curve? A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. And why is the equivalence point not always at ph7? In the beginning, the solution has a Everything you need to know. Titration Curve Explanation.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Explanation All acid titration curves follow the same basic shapes. [2,3,5] when interpreting a titration curve, it is essential to understand its key features. What are ph titration curves? It provides valuable information about the reaction under study and helps understand the results obtained. Both equivalence points are visible. Titration is a technique used in neutralisation reactions between acids and alkalis.. Titration Curve Explanation.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Curve Explanation The figure below shows two different. What are ph titration curves? How do you explain the shape of a titration curve? And why is the equivalence point not always at ph7? If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. Both. Titration Curve Explanation.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Explanation [2,3,5] when interpreting a titration curve, it is essential to understand its key features. What are ph titration curves? It provides valuable information about the reaction under study and helps understand the results obtained. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Both equivalence points are visible. All acid titration. Titration Curve Explanation.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curve Explanation And why is the equivalence point not always at ph7? A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps understand the results obtained. Both equivalence points are visible. In the beginning, the solution has a A typical titration curve of a diprotic. Titration Curve Explanation.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Explanation [2,3,5] when interpreting a titration curve, it is essential to understand its key features. It provides valuable information about the reaction under study and helps understand the results obtained. Both equivalence points are visible. The figure below shows two different. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Everything you. Titration Curve Explanation.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Curve Explanation In the beginning, the solution has a The figure below shows two different. It provides valuable information about the reaction under study and helps understand the results obtained. The equivalence point of a titration. All acid titration curves follow the same basic shapes. Titration is a technique used in neutralisation reactions between acids and alkalis. Both equivalence points are visible.. Titration Curve Explanation.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Curve Explanation If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. [2,3,5] when interpreting a titration curve, it is essential to understand its key features. The. Titration Curve Explanation.
From mungfali.com
Titration Curve Labeled Titration Curve Explanation Both equivalence points are visible. It provides valuable information about the reaction under study and helps understand the results obtained. [2,3,5] when interpreting a titration curve, it is essential to understand its key features. Everything you need to know for a level The figure below shows two different. What are ph titration curves? The titration curve is a graphical representation. Titration Curve Explanation.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curve Explanation It provides valuable information about the reaction under study and helps understand the results obtained. The equivalence point of a titration. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. A titration curve is a graphical representation of the ph of. Titration Curve Explanation.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Curve Explanation A titration curve is a graphical representation of the ph of a solution during a titration. All acid titration curves follow the same basic shapes. And why is the equivalence point not always at ph7? The figure below shows two different. It provides valuable information about the reaction under study and helps understand the results obtained. How do you explain. Titration Curve Explanation.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Explanation Both equivalence points are visible. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Everything you need to know for a level It provides valuable information about the reaction under study and helps understand the results obtained. A titration curve is a graphical representation of the ph of a solution during. Titration Curve Explanation.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Titration Curve Explanation The equivalence point of a titration. All acid titration curves follow the same basic shapes. And why is the equivalence point not always at ph7? Titration is a technique used in neutralisation reactions between acids and alkalis. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. It provides valuable information about the. Titration Curve Explanation.
From www.easybiologyclass.com
What is Titration Curve? What is pKa? EasyBiologyClass Titration Curve Explanation All acid titration curves follow the same basic shapes. Everything you need to know for a level The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Both equivalence points are visible. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. And why is. Titration Curve Explanation.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID7024887 Titration Curve Explanation How do you explain the shape of a titration curve? If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. Everything you need to know for a level The figure below shows two different. The equivalence point of a titration. A typical. Titration Curve Explanation.
From generalchemistrylab.blogspot.co.uk
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Curve Explanation And why is the equivalence point not always at ph7? A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In the beginning, the solution has a A titration curve is a graphical representation of the ph of a solution during a titration. What are ph titration curves? How do you explain the. Titration Curve Explanation.
From mavink.com
Strong Acid And Base Titration Curve Titration Curve Explanation And why is the equivalence point not always at ph7? A titration curve is a graphical representation of the ph of a solution during a titration. All acid titration curves follow the same basic shapes. It provides valuable information about the reaction under study and helps understand the results obtained. The equivalence point of a titration. What are ph titration. Titration Curve Explanation.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two Titration Curve Explanation All acid titration curves follow the same basic shapes. [2,3,5] when interpreting a titration curve, it is essential to understand its key features. The figure below shows two different. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Everything you need to know for a level Both equivalence points are visible. The. Titration Curve Explanation.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curve Explanation If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. The equivalence point of a titration. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. And why is the equivalence point not always. Titration Curve Explanation.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Explanation And why is the equivalence point not always at ph7? What are ph titration curves? [2,3,5] when interpreting a titration curve, it is essential to understand its key features. In the beginning, the solution has a The equivalence point of a titration. How do you explain the shape of a titration curve? Titration is a technique used in neutralisation reactions. Titration Curve Explanation.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Explanation If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. [2,3,5] when interpreting a titration curve, it is essential to understand its key features. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. It. Titration Curve Explanation.
From www.tessshebaylo.com
Karl Fischer Titration Equation Tessshebaylo Titration Curve Explanation [2,3,5] when interpreting a titration curve, it is essential to understand its key features. What are ph titration curves? It provides valuable information about the reaction under study and helps understand the results obtained. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a. Titration Curve Explanation.
From mungfali.com
Acid Titration Curve Titration Curve Explanation What are ph titration curves? The equivalence point of a titration. How do you explain the shape of a titration curve? The titration curve is a graphical representation of the ph or other property changes during a titration experiment. [2,3,5] when interpreting a titration curve, it is essential to understand its key features. If the ph of an acid solution. Titration Curve Explanation.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Explanation The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Everything you need to know for a level A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. It provides valuable information about the reaction under study and helps understand the results obtained. All acid. Titration Curve Explanation.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Curve Explanation What are ph titration curves? All acid titration curves follow the same basic shapes. How do you explain the shape of a titration curve? Everything you need to know for a level A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The equivalence point of a titration. And why is the equivalence. Titration Curve Explanation.
From www.youtube.com
Buffers and Titration Curves YouTube Titration Curve Explanation It provides valuable information about the reaction under study and helps understand the results obtained. Everything you need to know for a level The figure below shows two different. And why is the equivalence point not always at ph7? A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The titration curve is. Titration Curve Explanation.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Titration Curve Explanation [2,3,5] when interpreting a titration curve, it is essential to understand its key features. It provides valuable information about the reaction under study and helps understand the results obtained. Everything you need to know for a level The titration curve is a graphical representation of the ph or other property changes during a titration experiment. How do you explain the. Titration Curve Explanation.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Curve Explanation The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. Titration is a technique used in neutralisation reactions between acids and alkalis. [2,3,5] when interpreting a titration curve, it is essential to understand its key features. All acid titration curves follow the same basic shapes. The titration curve. Titration Curve Explanation.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Curve Explanation A titration curve is a graphical representation of the ph of a solution during a titration. Titration is a technique used in neutralisation reactions between acids and alkalis. The figure below shows two different. And why is the equivalence point not always at ph7? The equivalence point of a titration. A typical titration curve of a diprotic acid, oxalic acid,. Titration Curve Explanation.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Explanation How do you explain the shape of a titration curve? Titration is a technique used in neutralisation reactions between acids and alkalis. The equivalence point of a titration. It provides valuable information about the reaction under study and helps understand the results obtained. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.. Titration Curve Explanation.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Explanation In the beginning, the solution has a Everything you need to know for a level What are ph titration curves? If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. The figure below shows two different. It provides valuable information about the. Titration Curve Explanation.
From slidetodoc.com
Chapter 15 Redox Titrations 15 1 The Shape Titration Curve Explanation The equivalence point of a titration. Everything you need to know for a level Both equivalence points are visible. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. It provides valuable information about the reaction under study and helps understand the results obtained. What are ph titration curves? In the beginning,. Titration Curve Explanation.
From chem.libretexts.org
Complexation Titration Chemistry LibreTexts Titration Curve Explanation It provides valuable information about the reaction under study and helps understand the results obtained. The equivalence point of a titration. What are ph titration curves? A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. All acid titration curves follow the same basic shapes. [2,3,5] when interpreting a titration curve, it is. Titration Curve Explanation.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Explanation The figure below shows two different. It provides valuable information about the reaction under study and helps understand the results obtained. Everything you need to know for a level Titration is a technique used in neutralisation reactions between acids and alkalis. What are ph titration curves? If the ph of an acid solution is plotted against the amount of base. Titration Curve Explanation.