Why Is Heat Represented By Q . in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. the usual symbol for heat is ‘q’. Enthalpy is a function of state. The standard unit of heat in the international system of units (si) is the. heat released by a system into its surroundings is by convention a negative quantity (q < 0); Googling it shows many links but none of them seems convincent. why do we use $q$ for heat? If you know the state of a system,. according to this law, some heat given to the system is used to change the internal energy while the rest is used. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. enthalpy and heat are entirely different things.
from slidetodoc.com
Enthalpy is a function of state. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. The standard unit of heat in the international system of units (si) is the. Googling it shows many links but none of them seems convincent. If you know the state of a system,. the usual symbol for heat is ‘q’. according to this law, some heat given to the system is used to change the internal energy while the rest is used. why do we use $q$ for heat? in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. heat released by a system into its surroundings is by convention a negative quantity (q < 0);
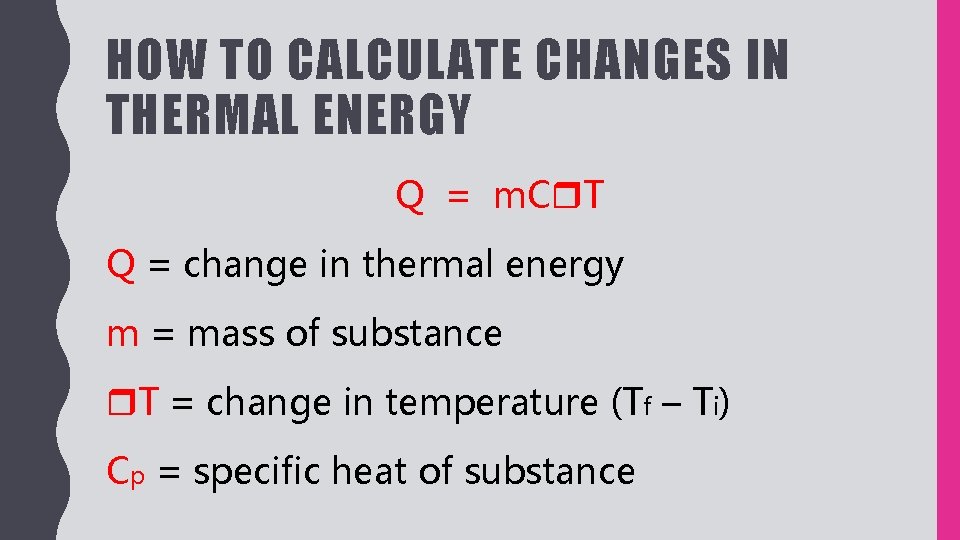
HEAT AND CALORIMETRY HEAT Represented by q Energy
Why Is Heat Represented By Q Enthalpy is a function of state. The standard unit of heat in the international system of units (si) is the. why do we use $q$ for heat? heat released by a system into its surroundings is by convention a negative quantity (q < 0); according to this law, some heat given to the system is used to change the internal energy while the rest is used. Enthalpy is a function of state. If you know the state of a system,. Googling it shows many links but none of them seems convincent. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. enthalpy and heat are entirely different things. the usual symbol for heat is ‘q’. in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present.
From slidetodoc.com
HEAT AND CALORIMETRY HEAT Represented by q Energy Why Is Heat Represented By Q The standard unit of heat in the international system of units (si) is the. If you know the state of a system,. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. why do we use $q$ for heat? the usual symbol for heat is ‘q’.. Why Is Heat Represented By Q.
From slidetodoc.com
Unit 3 Energy Part 2 Energy Transfer Chapter Why Is Heat Represented By Q according to this law, some heat given to the system is used to change the internal energy while the rest is used. If you know the state of a system,. Googling it shows many links but none of them seems convincent. in response to a reader query, this article traces the origins of the use of q and. Why Is Heat Represented By Q.
From www.noaa.gov
Heat exhaustion or heat stroke? Know the signs of heat illness Why Is Heat Represented By Q heat released by a system into its surroundings is by convention a negative quantity (q < 0); the usual symbol for heat is ‘q’. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. Googling it shows many links but none of them seems convincent. . Why Is Heat Represented By Q.
From www.doubtnut.com
Heat energy absorbed by a system in going through a cyclic process sho Why Is Heat Represented By Q heat released by a system into its surroundings is by convention a negative quantity (q < 0); Enthalpy is a function of state. enthalpy and heat are entirely different things. If you know the state of a system,. Googling it shows many links but none of them seems convincent. why do we use $q$ for heat? . Why Is Heat Represented By Q.
From www.youtube.com
Calculating Rate of Heat Transfer Between Two Working Fluids of a Heat Why Is Heat Represented By Q Googling it shows many links but none of them seems convincent. why do we use $q$ for heat? If you know the state of a system,. heat released by a system into its surroundings is by convention a negative quantity (q < 0); in response to a reader query, this article traces the origins of the use. Why Is Heat Represented By Q.
From www.youtube.com
Heat (w), Work (q), Change in Internal Energy (∆E) Practice Problems Why Is Heat Represented By Q Googling it shows many links but none of them seems convincent. The standard unit of heat in the international system of units (si) is the. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. in response to a reader query, this article traces the origins of. Why Is Heat Represented By Q.
From slidetodoc.com
HEAT AND CALORIMETRY HEAT Represented by q Energy Why Is Heat Represented By Q If you know the state of a system,. Googling it shows many links but none of them seems convincent. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. Enthalpy is a function of state. enthalpy and heat are entirely different things. in response to a. Why Is Heat Represented By Q.
From worksheetlibrarysteins.z19.web.core.windows.net
Formula To Calculate Specific Heat Why Is Heat Represented By Q the usual symbol for heat is ‘q’. heat released by a system into its surroundings is by convention a negative quantity (q < 0); according to this law, some heat given to the system is used to change the internal energy while the rest is used. why do we use $q$ for heat? If you know. Why Is Heat Represented By Q.
From slidetodoc.com
HEAT AND CALORIMETRY HEAT Represented by q Energy Why Is Heat Represented By Q the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. heat released by a system into its surroundings is by convention a negative quantity (q < 0); in response to a reader query, this article traces the origins of the use of q and q to. Why Is Heat Represented By Q.
From slideplayer.com
Thermochemistry. ppt download Why Is Heat Represented By Q the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. heat released by a system into its surroundings is by convention a negative quantity (q < 0); according to this law, some heat given to the system is used to change the internal energy while the. Why Is Heat Represented By Q.
From library.achievingthedream.org
Introduction to the Second Law of Thermodynamics Heat Engines and Why Is Heat Represented By Q according to this law, some heat given to the system is used to change the internal energy while the rest is used. enthalpy and heat are entirely different things. heat released by a system into its surroundings is by convention a negative quantity (q < 0); the usual symbol for heat is ‘q’. The standard unit. Why Is Heat Represented By Q.
From www.toppr.com
Three processes form a thermodynamic cycle as shown on P V diagram Why Is Heat Represented By Q The standard unit of heat in the international system of units (si) is the. in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is. Why Is Heat Represented By Q.
From www.grc.nasa.gov
Specific Heats Why Is Heat Represented By Q enthalpy and heat are entirely different things. heat released by a system into its surroundings is by convention a negative quantity (q < 0); why do we use $q$ for heat? If you know the state of a system,. the usual symbol for heat is ‘q’. Enthalpy is a function of state. the first law. Why Is Heat Represented By Q.
From slideplayer.com
Thermochemistry Heat and Chemical Change ppt download Why Is Heat Represented By Q why do we use $q$ for heat? If you know the state of a system,. heat released by a system into its surroundings is by convention a negative quantity (q < 0); Enthalpy is a function of state. Googling it shows many links but none of them seems convincent. the usual symbol for heat is ‘q’. . Why Is Heat Represented By Q.
From workshoprepairpuchernasx.z22.web.core.windows.net
Engine Schematic Diagram Why Is Heat Represented By Q enthalpy and heat are entirely different things. heat released by a system into its surroundings is by convention a negative quantity (q < 0); according to this law, some heat given to the system is used to change the internal energy while the rest is used. The standard unit of heat in the international system of units. Why Is Heat Represented By Q.
From infraredforhealth.com
How Do You Explain Negative Charge? Infrared for Health Why Is Heat Represented By Q the usual symbol for heat is ‘q’. heat released by a system into its surroundings is by convention a negative quantity (q < 0); If you know the state of a system,. The standard unit of heat in the international system of units (si) is the. enthalpy and heat are entirely different things. why do we. Why Is Heat Represented By Q.
From www.numerade.com
SOLVED P5.16 Convection heat transfer data are often reported as a Why Is Heat Represented By Q according to this law, some heat given to the system is used to change the internal energy while the rest is used. Enthalpy is a function of state. in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. heat released by. Why Is Heat Represented By Q.
From www.slideserve.com
PPT UNIT 5 THERMOCHEMISTRY 1 PowerPoint Presentation, free download Why Is Heat Represented By Q according to this law, some heat given to the system is used to change the internal energy while the rest is used. in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. If you know the state of a system,. the. Why Is Heat Represented By Q.
From tardigrade.in
A system is taken through a cyclic process represented by a circle as Why Is Heat Represented By Q Googling it shows many links but none of them seems convincent. why do we use $q$ for heat? the usual symbol for heat is ‘q’. enthalpy and heat are entirely different things. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. Enthalpy is a. Why Is Heat Represented By Q.
From answermediabrandt.z19.web.core.windows.net
Heat Transfer Specific Heat Problems Worksheet Why Is Heat Represented By Q in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. enthalpy and heat are entirely different things. heat released by a system into its surroundings is by convention a negative quantity (q < 0); The standard unit of heat in the. Why Is Heat Represented By Q.
From slidetodoc.com
HEAT AND CALORIMETRY HEAT Represented by q Energy Why Is Heat Represented By Q in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. enthalpy and heat are entirely different things. Googling it shows many links but none of them seems convincent. Enthalpy is a function of state. according to this law, some heat given. Why Is Heat Represented By Q.
From physics.stackexchange.com
thermodynamics Derivation of heat capacity at constant pressure and Why Is Heat Represented By Q according to this law, some heat given to the system is used to change the internal energy while the rest is used. Enthalpy is a function of state. The standard unit of heat in the international system of units (si) is the. Googling it shows many links but none of them seems convincent. enthalpy and heat are entirely. Why Is Heat Represented By Q.
From www.texasgateway.org
15.2 The First Law of Thermodynamics and Some Simple Processes Texas Why Is Heat Represented By Q Googling it shows many links but none of them seems convincent. according to this law, some heat given to the system is used to change the internal energy while the rest is used. the usual symbol for heat is ‘q’. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\). Why Is Heat Represented By Q.
From www.grc.nasa.gov
Heat Transfer Why Is Heat Represented By Q Enthalpy is a function of state. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. Googling it shows many links but none of them seems convincent. in response to a reader query, this article traces the origins of the use of q and q to symbolize. Why Is Heat Represented By Q.
From slidetodoc.com
HEAT AND CALORIMETRY HEAT Represented by q Energy Why Is Heat Represented By Q If you know the state of a system,. in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. Enthalpy is a function of state. Googling it shows many links but none of them seems convincent. why do we use $q$ for heat?. Why Is Heat Represented By Q.
From ch302.cm.utexas.edu
heating curve Why Is Heat Represented By Q Enthalpy is a function of state. according to this law, some heat given to the system is used to change the internal energy while the rest is used. Googling it shows many links but none of them seems convincent. enthalpy and heat are entirely different things. the usual symbol for heat is ‘q’. in response to. Why Is Heat Represented By Q.
From www.animalia-life.club
Specific Heat Chart Of Common Substances Why Is Heat Represented By Q enthalpy and heat are entirely different things. Enthalpy is a function of state. according to this law, some heat given to the system is used to change the internal energy while the rest is used. the usual symbol for heat is ‘q’. the first law of thermodynamics is given as \(\delta e = q + w\),. Why Is Heat Represented By Q.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Why Is Heat Represented By Q enthalpy and heat are entirely different things. If you know the state of a system,. heat released by a system into its surroundings is by convention a negative quantity (q < 0); why do we use $q$ for heat? in response to a reader query, this article traces the origins of the use of q and. Why Is Heat Represented By Q.
From studylib.net
Heat Equation Why Is Heat Represented By Q Enthalpy is a function of state. enthalpy and heat are entirely different things. The standard unit of heat in the international system of units (si) is the. heat released by a system into its surroundings is by convention a negative quantity (q < 0); the usual symbol for heat is ‘q’. according to this law, some. Why Is Heat Represented By Q.
From slidetodoc.com
HEAT AND CALORIMETRY HEAT Represented by q Energy Why Is Heat Represented By Q according to this law, some heat given to the system is used to change the internal energy while the rest is used. why do we use $q$ for heat? The standard unit of heat in the international system of units (si) is the. Googling it shows many links but none of them seems convincent. heat released by. Why Is Heat Represented By Q.
From nigerianscholars.com
Entropy and the Second Law of Thermodynamics Thermodynamics Why Is Heat Represented By Q heat released by a system into its surroundings is by convention a negative quantity (q < 0); the usual symbol for heat is ‘q’. enthalpy and heat are entirely different things. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. The standard unit of. Why Is Heat Represented By Q.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Why Is Heat Represented By Q Enthalpy is a function of state. the usual symbol for heat is ‘q’. in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. If you know the state of a system,. heat released by a system into its surroundings is by. Why Is Heat Represented By Q.
From learningzonegregorin2m.z4.web.core.windows.net
Heat Of Reaction Formula Why Is Heat Represented By Q why do we use $q$ for heat? the usual symbol for heat is ‘q’. The standard unit of heat in the international system of units (si) is the. Enthalpy is a function of state. in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834. Why Is Heat Represented By Q.
From www.tec-science.com
Specific latent heat of condensation tecscience Why Is Heat Represented By Q Googling it shows many links but none of them seems convincent. Enthalpy is a function of state. the first law of thermodynamics is given as \(\delta e = q + w\), where \(\delta e\) is the change in. in response to a reader query, this article traces the origins of the use of q and q to symbolize. Why Is Heat Represented By Q.
From studymind.co.uk
Heat Capacity Study Mind Why Is Heat Represented By Q heat released by a system into its surroundings is by convention a negative quantity (q < 0); why do we use $q$ for heat? the usual symbol for heat is ‘q’. in response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the. Why Is Heat Represented By Q.