Heat Of Evaporation Nitrogen . In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. thermal and mechanical properties of nitrogen p. = (1) where q is the amount of. Mannov department of reactor technology. vaporization of liquid nitrogen. Latent heat, l is defined to be: The input energy required to change the state. Table 6.4, heats of fusion, vaporization, and. As a system exchanges thermal energy with its surroundings, the temperature of the. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2).
from www.chegg.com
= (1) where q is the amount of. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. The input energy required to change the state. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Table 6.4, heats of fusion, vaporization, and. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. Mannov department of reactor technology. As a system exchanges thermal energy with its surroundings, the temperature of the. Latent heat, l is defined to be:
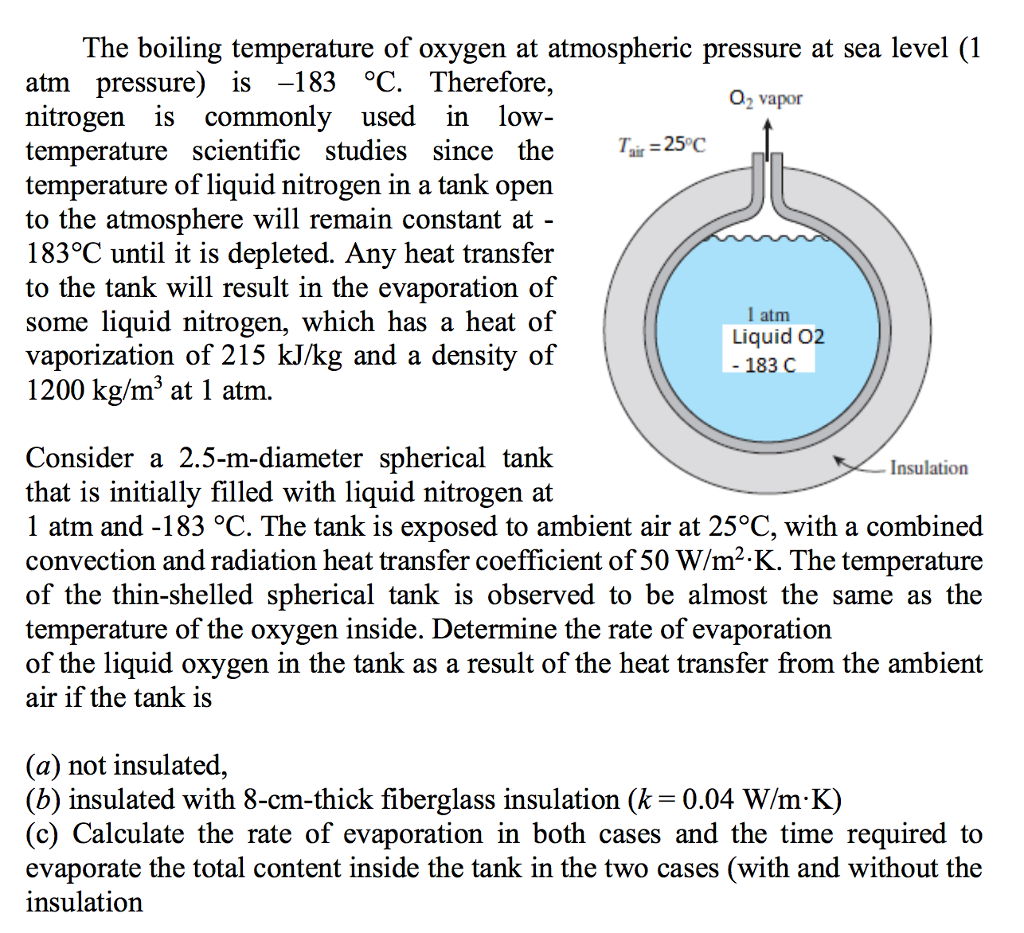
Solved The boiling temperature of oxygen at atmospheric
Heat Of Evaporation Nitrogen = (1) where q is the amount of. thermal and mechanical properties of nitrogen p. Mannov department of reactor technology. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. = (1) where q is the amount of. vaporization of liquid nitrogen. Latent heat, l is defined to be: latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). Table 6.4, heats of fusion, vaporization, and. The input energy required to change the state. As a system exchanges thermal energy with its surroundings, the temperature of the. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol.
From www.chegg.com
Solved The boiling temperature of nitrogen at atmospheric Heat Of Evaporation Nitrogen The input energy required to change the state. thermal and mechanical properties of nitrogen p. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. As a system exchanges thermal energy with its surroundings, the temperature of the. Table 6.4, heats of fusion, vaporization, and. the aim of the experiment was to determine the. Heat Of Evaporation Nitrogen.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Heat Of Evaporation Nitrogen As a system exchanges thermal energy with its surroundings, the temperature of the. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Mannov. Heat Of Evaporation Nitrogen.
From www.scribd.com
Experiment 1 Latent Heat of Liquid Nitrogen On Off PDF Heat Of Evaporation Nitrogen latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. The input energy required to change the state. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). latent. Heat Of Evaporation Nitrogen.
From www.chegg.com
Solved Determine the rate of evaporation (in kg/s) of the Heat Of Evaporation Nitrogen latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. The input energy required to change the state. Latent heat, l is defined to be: Table 6.4, heats of fusion, vaporization,. Heat Of Evaporation Nitrogen.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Heat Of Evaporation Nitrogen latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). = (1) where q is the amount of. As a system exchanges thermal energy with its surroundings, the temperature of the. latent heat of vaporization for fluids like alcohol, ether,. Heat Of Evaporation Nitrogen.
From www.periodic-table.org
Nitrogen Latent Heat of Vaporization Heat Of Evaporation Nitrogen latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. thermal and mechanical properties of nitrogen p. The input energy required to change the state. vaporization of liquid nitrogen. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol.. Heat Of Evaporation Nitrogen.
From www.chemix-chemistry-software.com
Nitrogen phase diagram Heat Of Evaporation Nitrogen As a system exchanges thermal energy with its surroundings, the temperature of the. The input energy required to change the state. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. vaporization of liquid nitrogen. thermal. Heat Of Evaporation Nitrogen.
From galvinconanstuart.blogspot.com
Nitrogen Phase Diagram Pressure Temperature General Wiring Diagram Heat Of Evaporation Nitrogen latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. Table 6.4, heats of fusion, vaporization, and. Latent heat, l is defined to be: the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). = (1) where. Heat Of Evaporation Nitrogen.
From www.youtube.com
What is Nitrogen Blowdown Evaporation Organomation YouTube Heat Of Evaporation Nitrogen Table 6.4, heats of fusion, vaporization, and. As a system exchanges thermal energy with its surroundings, the temperature of the. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. vaporization of liquid nitrogen. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. latent heat. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Vapour pressure curves of nitrogen and air. 1 airevaporation curve Heat Of Evaporation Nitrogen The input energy required to change the state. thermal and mechanical properties of nitrogen p. vaporization of liquid nitrogen. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). = (1) where q is the amount of. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. In case. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Pressureenthalpy diagram of nitrogen showing the evaporation Heat Of Evaporation Nitrogen vaporization of liquid nitrogen. The input energy required to change the state. Latent heat, l is defined to be: In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. thermal and mechanical properties of nitrogen p. Mannov department of reactor technology. = (1) where q is the amount of. As. Heat Of Evaporation Nitrogen.
From blog.organomation.com
Rules for Effective Nitrogen Blowdown Evaporation Heat Of Evaporation Nitrogen The input energy required to change the state. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). latent heat of vaporization. Heat Of Evaporation Nitrogen.
From olympiapublishers.com
Latent Heat Of Vaporization Of Liquid Nitrogen In J/g Heat Of Evaporation Nitrogen Table 6.4, heats of fusion, vaporization, and. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. Latent heat, l is defined to be: = (1) where q is the amount of. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. The input energy required to change the state. In case of liquid to. Heat Of Evaporation Nitrogen.
From democracyunlimited.web.fc2.com
heat of vaporization of liquid nitrogen Heat Of Evaporation Nitrogen vaporization of liquid nitrogen. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water. Heat Of Evaporation Nitrogen.
From www.britannica.com
How the Nitrogen Cycle Works Britannica Heat Of Evaporation Nitrogen Mannov department of reactor technology. = (1) where q is the amount of. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). As a system exchanges thermal energy with its surroundings, the temperature of the. Latent heat,. Heat Of Evaporation Nitrogen.
From golearning5.blogspot.com
The boiling temperature of nitrogen at atmospheric pressure at sea Heat Of Evaporation Nitrogen thermal and mechanical properties of nitrogen p. As a system exchanges thermal energy with its surroundings, the temperature of the. Table 6.4, heats of fusion, vaporization, and. = (1) where q is the amount of. The input energy required to change the state. vaporization of liquid nitrogen. the aim of the experiment was to determine the latent. Heat Of Evaporation Nitrogen.
From apollo.nvu.vsc.edu
Latent Heat of evaporation, fusion, and freezing Heat Of Evaporation Nitrogen thermal and mechanical properties of nitrogen p. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. vaporization of liquid nitrogen. Table 6.4, heats of fusion, vaporization, and. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. latent heat of vaporization for fluids like alcohol, ether, nitrogen,. Heat Of Evaporation Nitrogen.
From chemistry.about.com
Liquid Nitrogen Facts, Safety and Uses Heat Of Evaporation Nitrogen Latent heat, l is defined to be: the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. The input energy required to change the state. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. In case. Heat Of Evaporation Nitrogen.
From olympiapublishers.com
Latent Heat Of Vaporization Of Liquid Nitrogen In J/g Heat Of Evaporation Nitrogen The input energy required to change the state. As a system exchanges thermal energy with its surroundings, the temperature of the. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. Latent heat, l is defined to be: thermal and mechanical properties of nitrogen p. the aim of the experiment was to determine the. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Pressureenthalpy diagram of nitrogen showing the evaporation Heat Of Evaporation Nitrogen The input energy required to change the state. As a system exchanges thermal energy with its surroundings, the temperature of the. Table 6.4, heats of fusion, vaporization, and. vaporization of liquid nitrogen. = (1) where q is the amount of. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. In case of liquid to. Heat Of Evaporation Nitrogen.
From www.chegg.com
Solved 2. The boiling temperature of nitrogen at atmospheric Heat Of Evaporation Nitrogen latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). = (1) where q is the amount of. Mannov department of reactor technology. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. vaporization of liquid. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Pressureenthalpy diagram of nitrogen showing the evaporation Heat Of Evaporation Nitrogen vaporization of liquid nitrogen. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. The input energy required to change the state. Table 6.4, heats of fusion, vaporization, and. Mannov department of reactor technology. = (1) where q is the amount of. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and. Heat Of Evaporation Nitrogen.
From www.chegg.com
Solved Question 1 The boiling temperature of nitrogen at Heat Of Evaporation Nitrogen Table 6.4, heats of fusion, vaporization, and. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. As a system exchanges thermal energy with its surroundings, the temperature of the. The input energy required to change the state. = (1) where q is the amount of. Mannov department of reactor technology. latent heat of vaporization. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Oxygen, nitrogen and argon vapor pressure curves. Download Scientific Heat Of Evaporation Nitrogen The input energy required to change the state. = (1) where q is the amount of. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). Table 6.4, heats of fusion, vaporization, and. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. Latent heat, l is defined. Heat Of Evaporation Nitrogen.
From www.researchgate.net
A schematic diagram of the nitrogen reactive electron beam evaporation Heat Of Evaporation Nitrogen latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. = (1) where q is the amount of. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Latent heat, l is defined to be: latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. vaporization. Heat Of Evaporation Nitrogen.
From safetydata.netlify.app
What the boiling point of nitrogen in celsius safetydata Heat Of Evaporation Nitrogen The input energy required to change the state. As a system exchanges thermal energy with its surroundings, the temperature of the. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. = (1) where q is the amount of. Table 6.4, heats of fusion, vaporization, and. Latent heat, l is defined to be: latent heat of vaporization for. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Saturation curve for nitrogen. Download Scientific Diagram Heat Of Evaporation Nitrogen latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. Latent heat, l is defined to be: latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. vaporization of liquid nitrogen. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). Table 6.4, heats of fusion,. Heat Of Evaporation Nitrogen.
From www.chegg.com
Solved The boiling temperature of oxygen at atmospheric Heat Of Evaporation Nitrogen Latent heat, l is defined to be: Mannov department of reactor technology. vaporization of liquid nitrogen. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. = (1) where q is the amount of. Table 6.4, heats of fusion, vaporization,. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Nitrogen evaporation rate. Download Scientific Diagram Heat Of Evaporation Nitrogen Mannov department of reactor technology. Table 6.4, heats of fusion, vaporization, and. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. The input energy required to. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Pressureenthalpy diagram of nitrogen showing the evaporation Heat Of Evaporation Nitrogen Mannov department of reactor technology. vaporization of liquid nitrogen. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. The input energy required to change the state. As a system exchanges thermal energy with its surroundings, the temperature of the. Table 6.4, heats of fusion, vaporization, and. latent heat of vaporization for fluids like alcohol, ether, nitrogen,. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Pressureenthalpy diagram of nitrogen showing the evaporation Heat Of Evaporation Nitrogen latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. The input energy required to change the state. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. Latent heat, l is defined to be: As a system exchanges thermal energy. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Graph of liquid nitrogen volume (green line) and temperature of the Heat Of Evaporation Nitrogen In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Mannov department of reactor technology. The input energy required to change the state. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. the aim of. Heat Of Evaporation Nitrogen.
From www.researchgate.net
Saturation curve for nitrogen. Download Scientific Diagram Heat Of Evaporation Nitrogen In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. vaporization of liquid nitrogen. latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. Table 6.4, heats of fusion, vaporization, and. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. The input energy required to. Heat Of Evaporation Nitrogen.
From www.chegg.com
Solved The boiling temperature of nitrogen at atmospheric Heat Of Evaporation Nitrogen thermal and mechanical properties of nitrogen p. the aim of the experiment was to determine the latent heat of vaporisation of liquid nitrogen (ln2). latent heat of vaporization of nitrogen is (n2) 5.56 kj/mol. latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more. Table 6.4, heats of fusion, vaporization, and. latent. Heat Of Evaporation Nitrogen.
From elements.envato.com
Evaporation of heat from nitrogen rich soil fertilizer slow motion Heat Of Evaporation Nitrogen The input energy required to change the state. thermal and mechanical properties of nitrogen p. Latent heat, l is defined to be: In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Mannov department of reactor technology. vaporization of liquid nitrogen. As a system exchanges thermal energy with its surroundings,. Heat Of Evaporation Nitrogen.