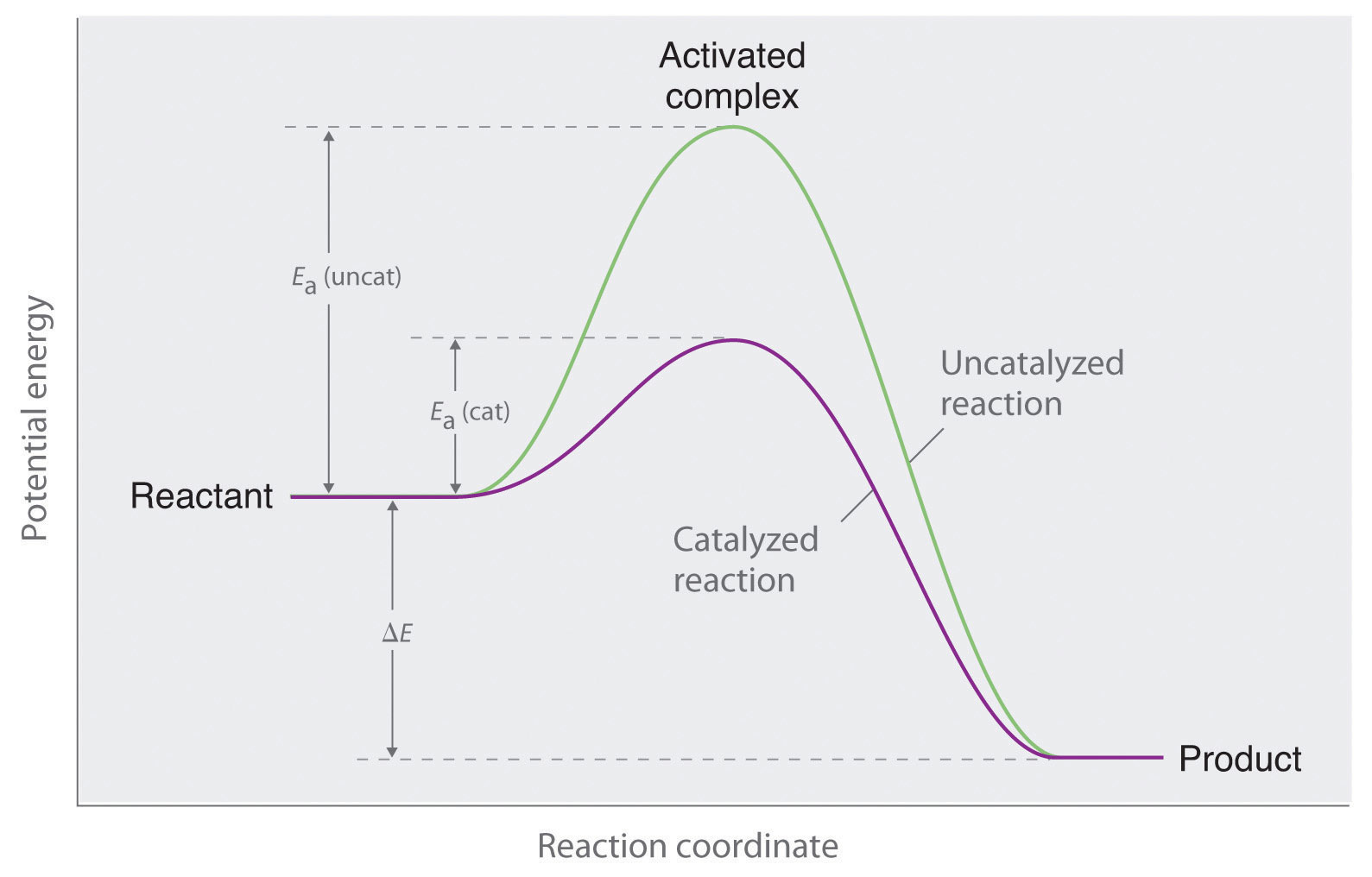
Catalyst Alter The Activation Energy Of . There is a subtle difference between the two statements that is easily illustrated with a simple analogy. a catalyst provides an alternative route for the reaction with a lower activation energy. It does not lower the activation energy of the reaction. A small quantity of catalyst should be able to affect the rate of reaction. catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. catalysts increase the rate of reaction. the key importance of activation energy. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation. generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. Catalysts are not consumed by the reaction. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate.
from 2012books.lardbucket.org
as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation. the key importance of activation energy. Catalysts are not consumed by the reaction. It does not lower the activation energy of the reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower.
Catalysis
Catalyst Alter The Activation Energy Of catalysts increase the rate of reaction. generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. the key importance of activation energy. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation. Catalysts are not consumed by the reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. A small quantity of catalyst should be able to affect the rate of reaction. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. It does not lower the activation energy of the reaction. catalysts increase the rate of reaction.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Alter The Activation Energy Of Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. generally, catalysts. Catalyst Alter The Activation Energy Of.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Catalyst Alter The Activation Energy Of It does not lower the activation energy of the reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. catalysts lower the activation energy (e a) of the reaction’s transition state by. Catalyst Alter The Activation Energy Of.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalyst Alter The Activation Energy Of catalysts increase the rate of reaction. generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. a catalyst provides an alternative route for the reaction with. Catalyst Alter The Activation Energy Of.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Catalyst Alter The Activation Energy Of as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. catalysts increase the rate of reaction. the key importance of activation energy. It does not lower the activation energy of the reaction. There is a subtle difference between the two statements that is easily illustrated with. Catalyst Alter The Activation Energy Of.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Alter The Activation Energy Of generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. Catalysts are not consumed by the reaction. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. a catalyst provides an alternative route for the reaction with. Catalyst Alter The Activation Energy Of.
From www.numerade.com
SOLVED 23. Complete the following statement A catalyst a) Increases Catalyst Alter The Activation Energy Of the key importance of activation energy. Catalysts are not consumed by the reaction. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. A catalyst may allow a reaction to proceed. Catalyst Alter The Activation Energy Of.
From 2012books.lardbucket.org
Catalysis Catalyst Alter The Activation Energy Of generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. as can be seen from the arrhenius equation, the magnitude of the activation. Catalyst Alter The Activation Energy Of.
From exozwuegv.blob.core.windows.net
Enzymes As Catalysts Ppt at Anthony Hubbard blog Catalyst Alter The Activation Energy Of catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. A small quantity of catalyst should be able to affect the rate of reaction. It does not lower the activation energy of the reaction. catalysts lower the activation energy (e a) of the reaction’s transition state by providing. Catalyst Alter The Activation Energy Of.
From telegra.ph
Catalyst activation energy diagram of enzymes Telegraph Catalyst Alter The Activation Energy Of There is a subtle difference between the two statements that is easily illustrated with a simple analogy. generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or. Catalyst Alter The Activation Energy Of.
From exoajjasl.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction From 20 To 10 at Catalyst Alter The Activation Energy Of It does not lower the activation energy of the reaction. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. the key importance of activation energy. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation. a catalyst provides. Catalyst Alter The Activation Energy Of.
From www.slideserve.com
PPT Activation Energy and Catalyst PowerPoint Presentation, free Catalyst Alter The Activation Energy Of the key importance of activation energy. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy. Catalyst Alter The Activation Energy Of.
From exoajjasl.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction From 20 To 10 at Catalyst Alter The Activation Energy Of catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. It does not lower the activation energy of the reaction. catalysts increase the rate of reaction.. Catalyst Alter The Activation Energy Of.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Alter The Activation Energy Of catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. the key importance of activation energy. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. a catalyst provides an alternative route for the reaction with a lower. Catalyst Alter The Activation Energy Of.
From www.tes.com
Catalysts and Activation Energy GCSE lesson (SC18c CC14c) Teaching Catalyst Alter The Activation Energy Of the key importance of activation energy. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. A small quantity of catalyst should be able to affect the rate of reaction. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy. Catalyst Alter The Activation Energy Of.
From www.researchgate.net
Activation energy for methane combustion with the two catalysts Catalyst Alter The Activation Energy Of There is a subtle difference between the two statements that is easily illustrated with a simple analogy. A small quantity of catalyst should be able to affect the rate of reaction. catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. Catalysts are not consumed by the reaction. . Catalyst Alter The Activation Energy Of.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalyst Alter The Activation Energy Of the key importance of activation energy. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. generally, catalysts alter the mechanism of the reaction in a substantial way such. Catalyst Alter The Activation Energy Of.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Alter The Activation Energy Of catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. a catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. generally, catalysts alter the. Catalyst Alter The Activation Energy Of.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Alter The Activation Energy Of A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. A small quantity of catalyst should be able to affect the rate of reaction. catalysts increase the rate of. Catalyst Alter The Activation Energy Of.
From wiredataisanewlk.z4.web.core.windows.net
How To Read Energy Diagrams Chemistry Catalyst Alter The Activation Energy Of catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. It does not lower the activation energy of the reaction. catalysts increase the rate of reaction. Catalysts are not consumed by the reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy,. Catalyst Alter The Activation Energy Of.
From sansona.github.io
Activation Energy Catalyst Alter The Activation Energy Of the key importance of activation energy. catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. as can be seen from the arrhenius equation, the magnitude of the. Catalyst Alter The Activation Energy Of.
From online-learning-college.com
Catalysts What is a catalyst? How do they work? Catalyst Alter The Activation Energy Of It does not lower the activation energy of the reaction. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation. A small quantity of catalyst should be able to affect the rate of reaction. the key importance of activation energy. A catalyst may allow a reaction to proceed at a lower. Catalyst Alter The Activation Energy Of.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalyst Alter The Activation Energy Of A small quantity of catalyst should be able to affect the rate of reaction. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. There is a subtle difference between. Catalyst Alter The Activation Energy Of.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Alter The Activation Energy Of the key importance of activation energy. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. generally, catalysts alter the mechanism of the reaction in. Catalyst Alter The Activation Energy Of.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Catalyst Alter The Activation Energy Of catalysts increase the rate of reaction. the key importance of activation energy. catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. A small quantity of catalyst should be able to affect the rate of reaction. There is a subtle difference between the two statements that is. Catalyst Alter The Activation Energy Of.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID1835084 Catalyst Alter The Activation Energy Of Catalysts are not consumed by the reaction. the key importance of activation energy. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. a catalyst provides an alternative route for the reaction with a lower activation energy. There is a subtle difference between the two statements. Catalyst Alter The Activation Energy Of.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Alter The Activation Energy Of the key importance of activation energy. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. It does not lower the activation energy of the reaction. generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. Catalysts. Catalyst Alter The Activation Energy Of.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst Alter The Activation Energy Of catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. A. Catalyst Alter The Activation Energy Of.
From kunduz.com
[ANSWERED] The addition of a catalyst during a chemical reaction alters Catalyst Alter The Activation Energy Of It does not lower the activation energy of the reaction. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. catalysts increase the rate of reaction. A catalyst may allow a. Catalyst Alter The Activation Energy Of.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalyst Alter The Activation Energy Of catalysts increase the rate of reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. catalysts lower the activation energy (e a) of the reaction’s transition state. Catalyst Alter The Activation Energy Of.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalyst Alter The Activation Energy Of a catalyst provides an alternative route for the reaction with a lower activation energy. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. It does not lower the activation energy of the reaction. A catalyst may allow a reaction to proceed at a lower temperature or. Catalyst Alter The Activation Energy Of.
From www.cheric.org
Chemical Reaction (Reaction rate) Catalyst Alter The Activation Energy Of There is a subtle difference between the two statements that is easily illustrated with a simple analogy. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate.. Catalyst Alter The Activation Energy Of.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Alter The Activation Energy Of catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation. the key importance of activation energy. generally, catalysts alter the mechanism of the reaction in a substantial way such. Catalyst Alter The Activation Energy Of.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Catalyst Alter The Activation Energy Of catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and different transition state. A small quantity of catalyst should be able to affect the rate of reaction. catalysts lower the activation energy (e a) of the reaction’s transition state by providing an alternative pathway for the reaction. Collisions only result in. Catalyst Alter The Activation Energy Of.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Alter The Activation Energy Of as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. catalysts permit an alternate mechanism for the reactants to become products, with. Catalyst Alter The Activation Energy Of.
From dxopopljn.blob.core.windows.net
Enzyme Catalyzed Reactions at Richard Fountain blog Catalyst Alter The Activation Energy Of Catalysts are not consumed by the reaction. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation. A small quantity of catalyst should be able to affect the rate of reaction. It does not lower the activation energy of the reaction. the key importance of activation energy. catalysts increase the. Catalyst Alter The Activation Energy Of.