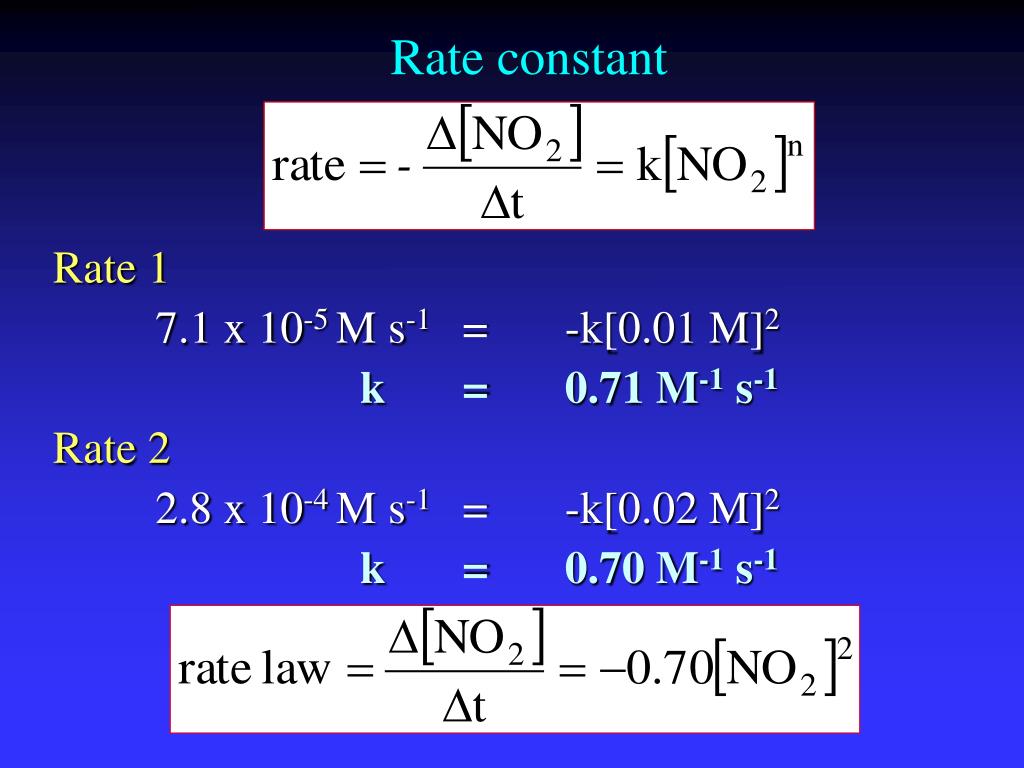
Is Rate Constant K Always Positive . The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Rate constants and the arrhenius equation. The negative sign is because the initial species $\ce{c}$ decays. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. Use rate laws to calculate reaction rates. So is negative, but the rate constant $k$ is always positive.
from www.slideserve.com
Explain the form and function of a rate law. Rate constants and the arrhenius equation. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. The negative sign is because the initial species $\ce{c}$ decays. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. So is negative, but the rate constant $k$ is always positive. Use rate and concentration data to identify reaction orders. Use rate laws to calculate reaction rates. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction.
PPT Chemical PowerPoint Presentation, free download ID6810274
Is Rate Constant K Always Positive The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. So is negative, but the rate constant $k$ is always positive. The negative sign is because the initial species $\ce{c}$ decays. The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. Explain the form and function of a rate law. Use rate laws to calculate reaction rates. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Use rate and concentration data to identify reaction orders. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. Rate constants and the arrhenius equation.
From pametno21.blogspot.com
K Constant Formula Chemistry pametno Is Rate Constant K Always Positive Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. Use rate laws to calculate reaction rates. The negative sign is because the initial species $\ce{c}$ decays. The equilibrium constant. Is Rate Constant K Always Positive.
From www.slideshare.net
Chemical Is Rate Constant K Always Positive So is negative, but the rate constant $k$ is always positive. The negative sign is because the initial species $\ce{c}$ decays. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. The exponents m and n are the reaction orders and are typically positive integers, though they can. Is Rate Constant K Always Positive.
From www.chegg.com
Solved What are the units of the rate constant (k) for a Is Rate Constant K Always Positive Use rate laws to calculate reaction rates. So is negative, but the rate constant $k$ is always positive. Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. The negative sign is because the initial species $\ce{c}$ decays. Rate constants and the arrhenius equation. The equilibrium constant is equal to the rate. Is Rate Constant K Always Positive.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation, free download ID Is Rate Constant K Always Positive Use rate and concentration data to identify reaction orders. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. So is negative, but the rate constant $k$ is always positive. Use rate laws to calculate reaction rates. The negative sign is because the initial species $\ce{c}$ decays. The rate. Is Rate Constant K Always Positive.
From www.researchgate.net
Estimation of reaction rate constants (K T ) Download Scientific Diagram Is Rate Constant K Always Positive This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. Use rate and concentration data to identify reaction orders. The exponents m and n are the reaction. Is Rate Constant K Always Positive.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID6810274 Is Rate Constant K Always Positive This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The negative sign is because the initial species $\ce{c}$ decays. Explain the form and function of a rate law. Rate constants and the arrhenius equation. Use rate laws to calculate reaction rates. The exponents m and n are the. Is Rate Constant K Always Positive.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Is Rate Constant K Always Positive Rate constants and the arrhenius equation. Explain the form and function of a rate law. The negative sign is because the initial species $\ce{c}$ decays. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Use rate and concentration data to identify reaction orders. This page looks at. Is Rate Constant K Always Positive.
From calculatorshub.net
Rate Constant K Calculator Online Is Rate Constant K Always Positive The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. Rate constants and the arrhenius equation. Use rate and concentration data to identify reaction orders. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Explain. Is Rate Constant K Always Positive.
From www.youtube.com
units of the rate constant k derivations YouTube Is Rate Constant K Always Positive The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. The negative sign is because the initial species $\ce{c}$ decays. This page looks at the way. Is Rate Constant K Always Positive.
From www.youtube.com
The Rate Constant k YouTube Is Rate Constant K Always Positive Use rate and concentration data to identify reaction orders. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. So is negative, but the rate constant $k$ is always positive. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate. Is Rate Constant K Always Positive.
From facts.net
14 Fascinating Facts About Rate Constant Is Rate Constant K Always Positive Use rate and concentration data to identify reaction orders. So is negative, but the rate constant $k$ is always positive. The negative sign is because the initial species $\ce{c}$ decays. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Explain the form and function of a rate. Is Rate Constant K Always Positive.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Is Rate Constant K Always Positive So is negative, but the rate constant $k$ is always positive. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. Use rate and concentration data to identify reaction orders. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant. Is Rate Constant K Always Positive.
From facts.net
13 Mindblowing Facts About Reaction Rate Constant Is Rate Constant K Always Positive This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The negative sign is because the initial species $\ce{c}$ decays. Use rate laws to calculate reaction rates. So is negative, but the rate constant $k$ is always positive. The rate constant, k, is a proportionality constant that links the. Is Rate Constant K Always Positive.
From www.researchgate.net
Arrhenius plots of the reaction rate constants k 1 and k 2 of reactions Is Rate Constant K Always Positive Explain the form and function of a rate law. The negative sign is because the initial species $\ce{c}$ decays. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a. Is Rate Constant K Always Positive.
From www.slideserve.com
PPT Rate laws (2) PowerPoint Presentation, free download ID2351800 Is Rate Constant K Always Positive So is negative, but the rate constant $k$ is always positive. Use rate laws to calculate reaction rates. Explain the form and function of a rate law. The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. The rate constant, k, is a proportionality constant that links the. Is Rate Constant K Always Positive.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Is Rate Constant K Always Positive This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The negative sign is because the initial species $\ce{c}$ decays. Rate constants and the arrhenius equation. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. So. Is Rate Constant K Always Positive.
From www.youtube.com
Reaction Rate vs Rate Constant Quick differences and comparison Is Rate Constant K Always Positive The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. So is negative, but the rate constant $k$ is always positive. The negative sign is because the. Is Rate Constant K Always Positive.
From slideplayer.com
Reaction Rates and Equilibrium ppt download Is Rate Constant K Always Positive Explain the form and function of a rate law. The negative sign is because the initial species $\ce{c}$ decays. Rate constants and the arrhenius equation. The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. The rate constant, k, is a proportionality constant that links the concentrations of. Is Rate Constant K Always Positive.
From general.chemistrysteps.com
Equilibrium Constant Chemistry Steps Is Rate Constant K Always Positive This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Explain the form and function of a rate law. The negative sign is because the initial species. Is Rate Constant K Always Positive.
From www.youtube.com
How to Determine Rate Expression and Rate Constant, k YouTube Is Rate Constant K Always Positive The negative sign is because the initial species $\ce{c}$ decays. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. Rate constants and the arrhenius equation.. Is Rate Constant K Always Positive.
From www.chegg.com
Solved What is the value of the rate constant k for this Is Rate Constant K Always Positive Use rate and concentration data to identify reaction orders. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Use rate laws to calculate reaction rates. So is negative, but the rate constant $k$ is always positive. Explain the form and function of a rate law. This page. Is Rate Constant K Always Positive.
From study.com
Rate Constant & Rate Law Definition, Differences & Examples Lesson Is Rate Constant K Always Positive So is negative, but the rate constant $k$ is always positive. The negative sign is because the initial species $\ce{c}$ decays. Use rate and concentration data to identify reaction orders. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. This page looks at the way that rate. Is Rate Constant K Always Positive.
From www.youtube.com
The rate constant `(K\')` of one reaction is double of the rate Is Rate Constant K Always Positive The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. Use rate laws to calculate reaction rates. So is negative, but the rate constant $k$ is. Is Rate Constant K Always Positive.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Is Rate Constant K Always Positive Explain the form and function of a rate law. Rate constants and the arrhenius equation. So is negative, but the rate constant $k$ is always positive. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The negative sign is because the initial species $\ce{c}$ decays. Use rate laws. Is Rate Constant K Always Positive.
From www.numerade.com
The rate constant (k) for a reaction is measured as a function of Is Rate Constant K Always Positive The negative sign is because the initial species $\ce{c}$ decays. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. This page looks at the way. Is Rate Constant K Always Positive.
From www.youtube.com
How to Determine Units of Rate Constant k (Shortcut with Examples Is Rate Constant K Always Positive Rate constants and the arrhenius equation. The negative sign is because the initial species $\ce{c}$ decays. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. So is negative, but the rate constant $k$ is always positive. The equilibrium constant is equal to the rate constant for the. Is Rate Constant K Always Positive.
From studylibplimsoll.z21.web.core.windows.net
What Is Constant Rate Is Rate Constant K Always Positive Use rate laws to calculate reaction rates. The negative sign is because the initial species $\ce{c}$ decays. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical. Is Rate Constant K Always Positive.
From studylibplimsoll.z21.web.core.windows.net
What Is Constant Rate Is Rate Constant K Always Positive The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. Rate constants and the arrhenius equation. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. Use rate and concentration data to identify reaction orders. So. Is Rate Constant K Always Positive.
From www.slideserve.com
PPT Today… PowerPoint Presentation, free download ID2134713 Is Rate Constant K Always Positive Explain the form and function of a rate law. Rate constants and the arrhenius equation. So is negative, but the rate constant $k$ is always positive. The negative sign is because the initial species $\ce{c}$ decays. Use rate and concentration data to identify reaction orders. This page looks at the way that rate constants vary with temperature and activation energy. Is Rate Constant K Always Positive.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Is Rate Constant K Always Positive So is negative, but the rate constant $k$ is always positive. The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. Use rate and concentration data to identify reaction orders. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate. Is Rate Constant K Always Positive.
From www.sliderbase.com
Determining Order with Concentration vs. Time data Is Rate Constant K Always Positive Use rate laws to calculate reaction rates. The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero. Explain the form and function of a rate law. The negative sign is because the initial species $\ce{c}$ decays. The rate constant, k, is a proportionality constant that links the concentrations. Is Rate Constant K Always Positive.
From www.storyofmathematics.com
Rate Constant Calculator + Online Solver With Free Steps Is Rate Constant K Always Positive Explain the form and function of a rate law. So is negative, but the rate constant $k$ is always positive. The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Use rate laws to calculate reaction rates. The exponents m and n are the reaction orders and are. Is Rate Constant K Always Positive.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID4095104 Is Rate Constant K Always Positive The negative sign is because the initial species $\ce{c}$ decays. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. The exponents m and n are the reaction orders and. Is Rate Constant K Always Positive.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Is Rate Constant K Always Positive So is negative, but the rate constant $k$ is always positive. Use rate and concentration data to identify reaction orders. Use rate laws to calculate reaction rates. The negative sign is because the initial species $\ce{c}$ decays. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The exponents. Is Rate Constant K Always Positive.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Is Rate Constant K Always Positive Use rate and concentration data to identify reaction orders. The negative sign is because the initial species $\ce{c}$ decays. The rate constant, k, is a proportionality constant that links the concentrations of certain species to the rate of a chemical reaction. This page looks at the way that rate constants vary with temperature and activation energy as shown by the. Is Rate Constant K Always Positive.