Aluminum Oxide Lattice Energy . The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. All other factors are the same. What is the formula of aluminum oxide? Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. Lattice dissociation enthalpies are always positive. The compound al 2 se 3 is used in the fabrication of some.
from www.chegg.com
The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. All other factors are the same. Lattice dissociation enthalpies are always positive. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. The compound al 2 se 3 is used in the fabrication of some. What is the formula of aluminum oxide? The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions.
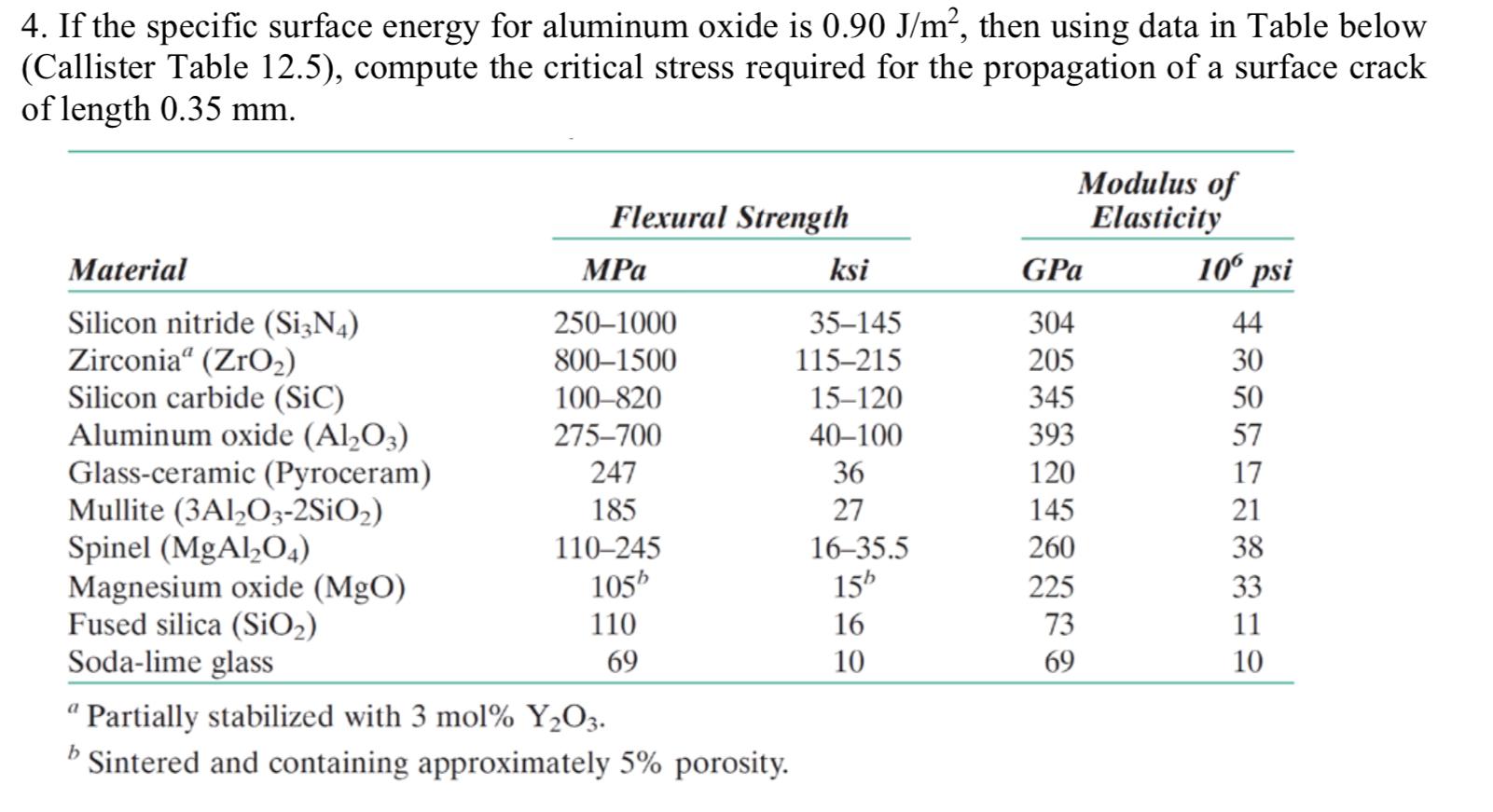
Solved 4. If the specific surface energy for aluminum oxide
Aluminum Oxide Lattice Energy The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. All other factors are the same. Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. The compound al 2 se 3 is used in the fabrication of some. What is the formula of aluminum oxide? The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Lattice dissociation enthalpies are always positive.
From www.slideserve.com
PPT Chapter 8 Concepts of Chemical Bonding PowerPoint Presentation Aluminum Oxide Lattice Energy Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. The compound al 2 se 3 is used in the fabrication of some. Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when. Aluminum Oxide Lattice Energy.
From fin3tutor.blogspot.com
How To Calculate Lattice Energy Of A Compound Aluminum Oxide Lattice Energy Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. The compound al 2 se 3 is used in the fabrication of some. All other factors are the same. Lattice dissociation enthalpies are always positive. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal. Aluminum Oxide Lattice Energy.
From www.pearson.com
The reaction of Fe2O3(s) with Al(s) to form Al2O3(s) and Fe(s) is Aluminum Oxide Lattice Energy Lattice dissociation enthalpies are always positive. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. All other factors are the same. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. What is the formula of aluminum oxide? Atoms. Aluminum Oxide Lattice Energy.
From www.youtube.com
Lattice Energy 6 Energy level diagram for Aluminium Oxide YouTube Aluminum Oxide Lattice Energy The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. What is the formula of aluminum oxide? Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its. Aluminum Oxide Lattice Energy.
From www.researchgate.net
Standard free energy of formation of metal oxides as a function of Aluminum Oxide Lattice Energy The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Lattice dissociation enthalpies are always positive. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. What is the formula of aluminum oxide? Lattice energy (chapter 19 tb) lattice energy is the enthalpy. Aluminum Oxide Lattice Energy.
From www.hindustanabrasives.com
Aluminium oxide (Al₂O₃) Definition, Structure, Properties Aluminum Oxide Lattice Energy The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. All other factors are the same. Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. The compound al. Aluminum Oxide Lattice Energy.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Aluminium Oxide Lattice Constant Aluminum Oxide Lattice Energy The compound al 2 se 3 is used in the fabrication of some. The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. All. Aluminum Oxide Lattice Energy.
From www.semanticscholar.org
[PDF] Crystal Structure of Alumina Oxide and Hydroxide Phases . ( After Aluminum Oxide Lattice Energy Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. What is the formula of aluminum oxide? Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. Atoms can come together in many different ways, and this. Aluminum Oxide Lattice Energy.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Aluminium Oxide Lattice Constant Aluminum Oxide Lattice Energy Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. The compound al 2 se 3 is used in the fabrication of some. What is. Aluminum Oxide Lattice Energy.
From www.semanticscholar.org
Figure 6 from Synthesis of γalumina nanoparticles by wireexplosion Aluminum Oxide Lattice Energy The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. What is the formula of aluminum oxide? The compound al 2 se 3 is used in the fabrication of some.. Aluminum Oxide Lattice Energy.
From www.youtube.com
Born Haber Cycle Part 2 Aluminium Oxide Al2O3 YouTube Aluminum Oxide Lattice Energy The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. The compound al 2. Aluminum Oxide Lattice Energy.
From www.linstitute.net
CIE A Level Chemistry复习笔记5.1.5 Factors Affecting Lattice Energy翰林国际教育 Aluminum Oxide Lattice Energy Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. What is the formula of aluminum. Aluminum Oxide Lattice Energy.
From scoop.eduncle.com
The lattice energy of lif calculated from bornlande equation 1000 kj Aluminum Oxide Lattice Energy The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. The compound al 2 se 3 is used in the fabrication of some. What is the formula of aluminum oxide? All other factors are the same. Atoms can come together in many different ways, and this lattice energy calculator. Aluminum Oxide Lattice Energy.
From fin3tutor.blogspot.com
How To Calculate Lattice Energy Of A Compound Aluminum Oxide Lattice Energy Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. Lattice dissociation enthalpies are always positive. The compound al 2 se. Aluminum Oxide Lattice Energy.
From www.youtube.com
Born Haber Cycle Aluminium Oxide (Al2O3) YouTube Aluminum Oxide Lattice Energy Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. Lattice dissociation enthalpies are always positive. All other factors are the same. What is the formula of. Aluminum Oxide Lattice Energy.
From www.researchgate.net
JT distortion in 3D and 2D oxide lattices a, Schematic MO6 (where M is Aluminum Oxide Lattice Energy Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. The compound al 2 se 3 is used in the fabrication of some. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. Lattice dissociation. Aluminum Oxide Lattice Energy.
From www.chegg.com
Solved Determine the lattice energy for aluminum oxide using Aluminum Oxide Lattice Energy Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. What is the formula of aluminum oxide? The compound al 2 se 3 is used in the fabrication of some.. Aluminum Oxide Lattice Energy.
From www.researchgate.net
Crystal structure of aluminum oxide (Al 2 O 3 ) (a) top view and (b Aluminum Oxide Lattice Energy Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. All other factors are the same. The compound al 2 se 3 is used in the fabrication of some. Lattice energy (chapter 19 tb) lattice energy is the enthalpy change. Aluminum Oxide Lattice Energy.
From webmis.highland.cc.il.us
Lattice Energies in Ionic Solids Aluminum Oxide Lattice Energy Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. The compound al 2 se 3 is used in. Aluminum Oxide Lattice Energy.
From www.researchgate.net
(PDF) Standard enthalpy and gibbs energy of formation of crystalline Aluminum Oxide Lattice Energy What is the formula of aluminum oxide? Lattice dissociation enthalpies are always positive. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. The compound al 2 se 3 is used in the fabrication of some. Atoms can come together in many different ways, and this lattice energy calculator is concerned. Aluminum Oxide Lattice Energy.
From www.chegg.com
Solved 4. If the specific surface energy for aluminum oxide Aluminum Oxide Lattice Energy Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. The compound al 2 se 3 is used in the fabrication of some. What is the formula of aluminum oxide? Na2o has the higher lattice energy than naoh because the. Aluminum Oxide Lattice Energy.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (6) More on Lattice Energy Aluminum Oxide Lattice Energy All other factors are the same. Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. Lattice dissociation enthalpies are always positive. What is the formula of. Aluminum Oxide Lattice Energy.
From chem.libretexts.org
Chapter 4.2 Lattice Energies in Ionic Solids Chemistry LibreTexts Aluminum Oxide Lattice Energy What is the formula of aluminum oxide? Lattice dissociation enthalpies are always positive. Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into. Aluminum Oxide Lattice Energy.
From people.wou.edu
Physical State and Structures of Oxides Aluminum Oxide Lattice Energy The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of. Aluminum Oxide Lattice Energy.
From www.science.org
Orbital Physics in TransitionMetal Oxides Science Aluminum Oxide Lattice Energy Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. Lattice dissociation enthalpies are always positive. The compound al 2 se 3 is used in. Aluminum Oxide Lattice Energy.
From www2.mdpi.com
Crystals Free FullText Phase Properties of Different HfO2 Aluminum Oxide Lattice Energy The compound al 2 se 3 is used in the fabrication of some. Lattice dissociation enthalpies are always positive. The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. The. Aluminum Oxide Lattice Energy.
From inrikoloco.weebly.com
Lattice energy trend periodic table compounds inrikoloco Aluminum Oxide Lattice Energy Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. The compound al 2 se 3 is used in the fabrication of some. Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic. Aluminum Oxide Lattice Energy.
From webmis.highland.cc.il.us
Lattice Energies in Ionic Solids Aluminum Oxide Lattice Energy Lattice energy (chapter 19 tb) lattice energy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into. Aluminum Oxide Lattice Energy.
From www.youtube.com
Lattice Enthalpy for Aluminium Oxide YouTube Aluminum Oxide Lattice Energy The compound al 2 se 3 is used in the fabrication of some. The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. The lattice dissociation enthalpy is the enthalpy change needed to convert 1. Aluminum Oxide Lattice Energy.
From woodall.ece.ucdavis.edu
Resources Aluminum Oxide Lattice Energy Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. Lattice dissociation enthalpies are always positive. The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. All other factors are the same. The compound al 2 se 3 is used in the fabrication of some.. Aluminum Oxide Lattice Energy.
From www.researchgate.net
Ellingham diagram showing the standard Gibbs energies of formation of Aluminum Oxide Lattice Energy Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when cations and anions ionically bond as a part of a. The compound al 2 se 3 is used in the fabrication of some. The precious gem ruby is aluminum oxide, al 2 o 3, containing traces of cr 3+. Lattice. Aluminum Oxide Lattice Energy.
From www.semanticscholar.org
Figure 1 from Crystal Structure of Alumina Oxide and Hydroxide Phases Aluminum Oxide Lattice Energy The compound al 2 se 3 is used in the fabrication of some. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. Atoms can come together in many different. Aluminum Oxide Lattice Energy.
From www.chegg.com
Solved 20. Using the data below, calculate the lattice Aluminum Oxide Lattice Energy The compound al 2 se 3 is used in the fabrication of some. All other factors are the same. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. Atoms can come together in many different ways, and this lattice energy calculator is concerned with the energy stored when. Aluminum Oxide Lattice Energy.
From www.researchgate.net
Crystal structures of metal oxides [20] Download Scientific Diagram Aluminum Oxide Lattice Energy Na2o has the higher lattice energy than naoh because the charge of o2− is larger than that of oh−. What is the formula of aluminum oxide? All other factors are the same. Lattice dissociation enthalpies are always positive. The compound al 2 se 3 is used in the fabrication of some. The lattice dissociation enthalpy is the enthalpy change needed. Aluminum Oxide Lattice Energy.
From www.numerade.com
SOLVED Using the data below, calculate the lattice energy of sodium Aluminum Oxide Lattice Energy All other factors are the same. What is the formula of aluminum oxide? The compound al 2 se 3 is used in the fabrication of some. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. Lattice dissociation enthalpies are always positive. Lattice energy (chapter 19 tb) lattice energy. Aluminum Oxide Lattice Energy.