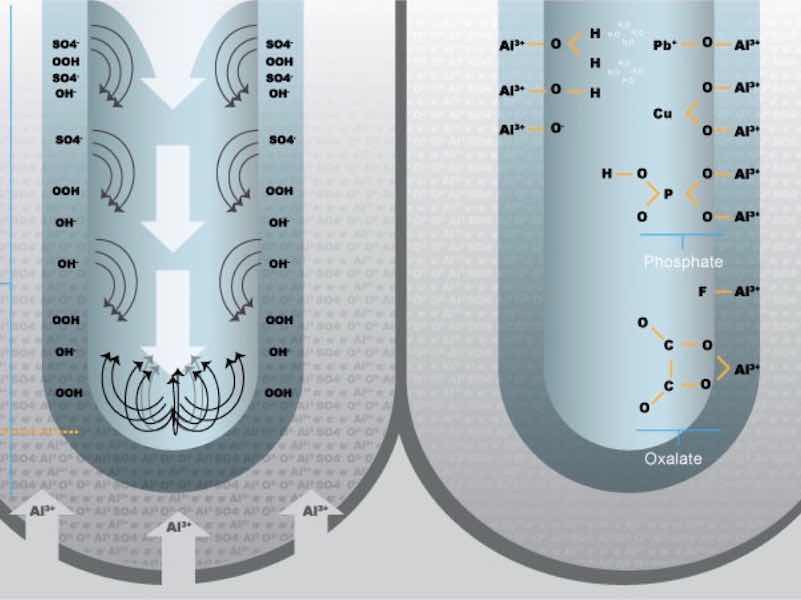
Aluminum Oxide Anion . Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. We know that cobalt can have more than one possible charge; For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The name of this ionic compound is aluminum fluoride. Aluminium oxide reacts with hot dilute hydrochloric acid to give. Change the oxygen ending to the. We just need to determine.
from finishingandcoating.com
For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. We know that cobalt can have more than one possible charge; The name of this ionic compound is aluminum fluoride. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric acid to give. Change the oxygen ending to the. We just need to determine. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change.
Enhancing Anodic Aluminum Oxide for Bonding Applications
Aluminum Oxide Anion Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. We know that cobalt can have more than one possible charge; Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric acid to give. Change the oxygen ending to the. We just need to determine. The name of this ionic compound is aluminum fluoride. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change.
From www.slideserve.com
PPT 1 Name the ions formed by these elements and classify them as anions or cations Aluminum Oxide Anion Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. We know that cobalt can have more than one possible charge; Change the oxygen ending to the. Aluminium oxide reacts with hot dilute hydrochloric acid to give. We just need to determine. Aluminium oxide contains oxide ions, and thus reacts with acids. Aluminum Oxide Anion.
From www.goodscience.com.au
Determining the Formula for Ionic Compounds Good Science Aluminum Oxide Anion Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Change the oxygen ending to the. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. We just need to determine. We know that cobalt can have. Aluminum Oxide Anion.
From blog.thepipingmart.com
How to Identify Aluminium Oxide An Overview Aluminum Oxide Anion Change the oxygen ending to the. The name of this ionic compound is aluminum fluoride. We just need to determine. Aluminium oxide reacts with hot dilute hydrochloric acid to give. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide contains oxide ions, and thus reacts with acids in the. Aluminum Oxide Anion.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID2031508 Aluminum Oxide Anion We know that cobalt can have more than one possible charge; For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. We just need to determine. Change the oxygen ending to the. Al is a type i monatomic ion, no roman numeral is needed since. Aluminum Oxide Anion.
From stonecoldhands.com
Ionic Compounds Aluminum Oxide Anion We know that cobalt can have more than one possible charge; Change the oxygen ending to the. We just need to determine. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations,. Aluminum Oxide Anion.
From study.com
Aluminum Oxide Compound Formula, Properties & Structure Video & Lesson Transcript Aluminum Oxide Anion Aluminium oxide reacts with hot dilute hydrochloric acid to give. Change the oxygen ending to the. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. We know that cobalt can have more than one possible charge; The name of this ionic compound is aluminum fluoride. Al is a type i. Aluminum Oxide Anion.
From achs-prod.acs.org
GasPhase Fragmentation of Aluminum Oxide Nitrate Anions Driven by Reactive Oxygen Radical Aluminum Oxide Anion For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. The name of this ionic compound is aluminum fluoride. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Change the oxygen ending to the. We know. Aluminum Oxide Anion.
From digital.library.unt.edu
Effect of Anion, Ph, and Temperature on the Dissolution Behavior of Aluminum Oxide Films. Page Aluminum Oxide Anion Aluminium oxide reacts with hot dilute hydrochloric acid to give. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Change the oxygen ending to the. We. Aluminum Oxide Anion.
From testbook.com
Aluminium Oxide Learn Definition, Structure, Properties, And Uses Aluminum Oxide Anion The name of this ionic compound is aluminum fluoride. Change the oxygen ending to the. We just need to determine. We know that cobalt can have more than one possible charge; Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. For example, the formula for aluminum oxide, al 2 o. Aluminum Oxide Anion.
From www.researchgate.net
Anion exchange membrane with wellordered arrays of ionic channels based on a porous anodic Aluminum Oxide Anion The name of this ionic compound is aluminum fluoride. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide reacts with hot dilute hydrochloric acid to give. We just need to determine. Change the oxygen ending to the. For example, the formula for aluminum oxide, al 2 o 3, indicates. Aluminum Oxide Anion.
From www.slideserve.com
PPT 1 Name the ions formed by these elements and classify them as anions or cations Aluminum Oxide Anion Aluminium oxide reacts with hot dilute hydrochloric acid to give. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. We know that cobalt can have more than one possible charge; Change the. Aluminum Oxide Anion.
From finishingandcoating.com
Enhancing Anodic Aluminum Oxide for Bonding Applications Aluminum Oxide Anion Aluminium oxide reacts with hot dilute hydrochloric acid to give. We know that cobalt can have more than one possible charge; Change the oxygen ending to the. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide contains oxide ions, and thus reacts. Aluminum Oxide Anion.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID3061831 Aluminum Oxide Anion We know that cobalt can have more than one possible charge; Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. We just need to determine. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric. Aluminum Oxide Anion.
From www.semanticscholar.org
Figure 1 from GasPhase Vibrational Spectroscopy of the Aluminum Oxide Anions (Al2 O3 )16 AlO2 Aluminum Oxide Anion Aluminium oxide reacts with hot dilute hydrochloric acid to give. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. We know that cobalt can have more than one possible charge; The name of this ionic compound is aluminum fluoride. For example, the formula for aluminum oxide, al 2 o 3, indicates. Aluminum Oxide Anion.
From www.youtube.com
Give formation of Aluminium oxide Formation of aluminium oxide by transfer of electrons YouTube Aluminum Oxide Anion Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide reacts with hot dilute hydrochloric acid to give. The name of this ionic compound is aluminum fluoride. We know that cobalt can have more than one possible charge; We just need to determine. For example, the formula for aluminum oxide,. Aluminum Oxide Anion.
From www.slideshare.net
Ch 8 ionic compounds Aluminum Oxide Anion Aluminium oxide reacts with hot dilute hydrochloric acid to give. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Al is a type i monatomic. Aluminum Oxide Anion.
From pubs.acs.org
Catalytic NO Reduction by NobleMetalFree VanadiumAluminum Oxide Cluster Anions The Journal Aluminum Oxide Anion Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. Aluminum Oxide Anion.
From www.researchgate.net
Schematic twodimensional representation of aluminum oxide structures. Download Scientific Diagram Aluminum Oxide Anion Change the oxygen ending to the. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. We just need to determine. We know that cobalt can have more than one possible charge; Aluminium oxide reacts with hot dilute hydrochloric acid to give. Aluminium oxide contains oxide ions, and thus reacts with acids. Aluminum Oxide Anion.
From www.youtube.com
How to write the equation for Al2O3 + H2O (Aluminum oxide + Water) YouTube Aluminum Oxide Anion Change the oxygen ending to the. The name of this ionic compound is aluminum fluoride. We just need to determine. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium. Aluminum Oxide Anion.
From chemistry291.blogspot.com
What Is the Chemical Formula for Aluminum Oxide? Aluminum Oxide Anion Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. We know that cobalt can have more than one possible charge; For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. We just need to determine. Aluminium. Aluminum Oxide Anion.
From www.semanticscholar.org
Figure 1 from GasPhase Vibrational Spectroscopy of the Aluminum Oxide Anions (Al2 O3 )16 AlO2 Aluminum Oxide Anion Change the oxygen ending to the. Aluminium oxide reacts with hot dilute hydrochloric acid to give. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. The name of this ionic compound is aluminum fluoride. Aluminium oxide contains oxide ions, and thus reacts with acids. Aluminum Oxide Anion.
From www.semanticscholar.org
Figure 1 from Anion photoelectron spectroscopy and density functional study of small aluminum Aluminum Oxide Anion Aluminium oxide reacts with hot dilute hydrochloric acid to give. We know that cobalt can have more than one possible charge; For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way. Aluminum Oxide Anion.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Formula For Aluminium Oxide Aluminum Oxide Anion We know that cobalt can have more than one possible charge; We just need to determine. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide reacts with hot dilute hydrochloric acid to give. Change the oxygen ending to the. For example, the formula for aluminum oxide, al 2 o. Aluminum Oxide Anion.
From chemistry-europe.onlinelibrary.wiley.com
Theoretical Study on Aluminum Oxide Cluster Anions A12Ox− (x=2−5) with Rhombus Structure Junxi Aluminum Oxide Anion Change the oxygen ending to the. We just need to determine. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric acid to give. We know. Aluminum Oxide Anion.
From www.pw.live
Aluminium Oxide Formula, Structure, Properties, Uses Aluminum Oxide Anion Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The name of this ionic compound is aluminum fluoride. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Change the oxygen ending to the. We. Aluminum Oxide Anion.
From karsyntinoconnell.blogspot.com
Cations and Anions List KarsyntinOconnell Aluminum Oxide Anion We know that cobalt can have more than one possible charge; The name of this ionic compound is aluminum fluoride. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Change the oxygen. Aluminum Oxide Anion.
From sciencenotes.org
Common Anions List and Formulas Aluminum Oxide Anion Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric acid to give. We just need to determine. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. The. Aluminum Oxide Anion.
From www.youtube.com
Write the chemical formula of Aluminium oxide YouTube Aluminum Oxide Anion Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. We just need to determine. Aluminium oxide reacts with hot dilute hydrochloric acid to give. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Change. Aluminum Oxide Anion.
From www.semanticscholar.org
Figure 2 from GasPhase Vibrational Spectroscopy of the Aluminum Oxide Anions (Al2 O3 )16 AlO2 Aluminum Oxide Anion Change the oxygen ending to the. The name of this ionic compound is aluminum fluoride. We just need to determine. We know that cobalt can have more than one possible charge; For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Al is a type. Aluminum Oxide Anion.
From www.researchgate.net
Aluminium can promote Fenton reaction through the following cycle i)... Download Scientific Aluminum Oxide Anion Aluminium oxide reacts with hot dilute hydrochloric acid to give. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. For example, the formula for aluminum oxide, al 2 o 3, indicates that. Aluminum Oxide Anion.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Aluminum Oxide Anion Aluminium oxide reacts with hot dilute hydrochloric acid to give. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. The name of this ionic compound. Aluminum Oxide Anion.
From www.toppr.com
Aluminium Oxide Formula Chemical Formula, Properties, Applications Aluminum Oxide Anion We just need to determine. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide reacts with hot dilute hydrochloric acid to give. Aluminium oxide. Aluminum Oxide Anion.
From www.youtube.com
Lewis Dot Structure for Al2O3 (Aluminum Oxide) YouTube Aluminum Oxide Anion For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide reacts with hot dilute hydrochloric acid to give. We know that cobalt can have more than one possible charge; Aluminium oxide contains oxide ions, and thus reacts with acids in the same way. Aluminum Oxide Anion.
From www.youtube.com
Is Al2O3 (Aluminum oxide) Ionic or Covalent? YouTube Aluminum Oxide Anion Change the oxygen ending to the. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide reacts with hot dilute hydrochloric acid to give. The name of this ionic compound is aluminum fluoride. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium. Aluminum Oxide Anion.
From chemistry-europe.onlinelibrary.wiley.com
Inside Cover Gas‐Phase Vibrational Spectroscopy of the Aluminum Oxide Anions (Al2O3)16AlO2− Aluminum Oxide Anion The name of this ionic compound is aluminum fluoride. We know that cobalt can have more than one possible charge; Aluminium oxide reacts with hot dilute hydrochloric acid to give. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. We just need to determine. Change the oxygen ending to the.. Aluminum Oxide Anion.