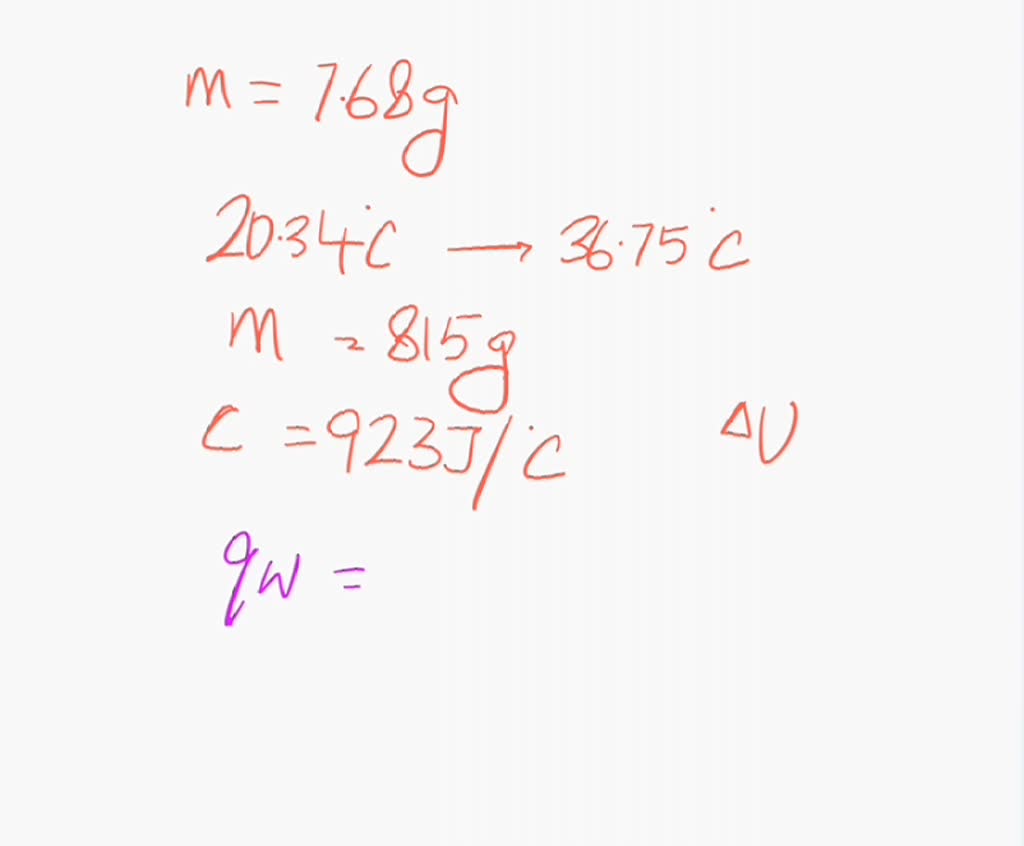Bomb Calorimeter Has A Heat Capacity . In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the calorimeter which is later. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Four critical parts are needed in every bomb calorimeter.
from www.numerade.com
Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. Four critical parts are needed in every bomb calorimeter. Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the calorimeter which is later. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. The heat capacity of the bomb calorimeter is 2.36 kj/ °c.
SOLVED A bomb calorimeter has a heat capacity of 675 J/PC and contains
Bomb Calorimeter Has A Heat Capacity The heat capacity of the bomb calorimeter is 2.36 kj/ °c. Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the calorimeter which is later. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Four critical parts are needed in every bomb calorimeter. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Bomb Calorimeter Has A Heat Capacity Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\). Bomb Calorimeter Has A Heat Capacity.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Bomb Calorimeter Has A Heat Capacity Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Four critical parts are needed in every bomb calorimeter. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). The heat capacity of the bomb calorimeter is 2.36 kj/ °c. Heat. Bomb Calorimeter Has A Heat Capacity.
From www.slideserve.com
PPT Introduction to Thermochemistry PowerPoint Presentation, free Bomb Calorimeter Has A Heat Capacity Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Four critical parts are needed in every bomb calorimeter. Observation of the temperature rise caused. Bomb Calorimeter Has A Heat Capacity.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter Has A Heat Capacity Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. Four critical parts are needed in every bomb calorimeter. Calculate the amount of heat released during the combustion of glucose. Bomb Calorimeter Has A Heat Capacity.
From shaunmwilliams.com
Chapter 6 Presentation Bomb Calorimeter Has A Heat Capacity Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the calorimeter which is later. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g). Bomb Calorimeter Has A Heat Capacity.
From www.chegg.com
Solved A bomb calorimeter has a heat capacity of 2.47 kJ/K. Bomb Calorimeter Has A Heat Capacity Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the. Bomb Calorimeter Has A Heat Capacity.
From byjus.com
What is bomb calorimeter? Bomb Calorimeter Has A Heat Capacity When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Four critical parts are needed in every bomb calorimeter. 2c 4 h 10 (g). Bomb Calorimeter Has A Heat Capacity.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Bomb Calorimeter Has A Heat Capacity Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. In chemistry, the changes of heat of a reaction can be. Bomb Calorimeter Has A Heat Capacity.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Has A Heat Capacity 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the. Bomb Calorimeter Has A Heat Capacity.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Has A Heat Capacity Four critical parts are needed in every bomb calorimeter. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h. Bomb Calorimeter Has A Heat Capacity.
From www.labster.com
5 Ways to Make Calorimetry and the Bomb Calorimeter More Approachable Bomb Calorimeter Has A Heat Capacity Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)). Bomb Calorimeter Has A Heat Capacity.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Bomb Calorimeter Has A Heat Capacity Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the calorimeter which is later. Four critical parts are needed in every bomb calorimeter. In chemistry, the changes. Bomb Calorimeter Has A Heat Capacity.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Has A Heat Capacity Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Observation of the temperature rise caused by this amount of heat enables us. Bomb Calorimeter Has A Heat Capacity.
From people.chem.umass.edu
to Adobe GoLive 6 Bomb Calorimeter Has A Heat Capacity Four critical parts are needed in every bomb calorimeter. Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Calculate the amount of heat released during the combustion. Bomb Calorimeter Has A Heat Capacity.
From www.shutterstock.com
Bomb Calorimeter Vector Illustration Labeled Educational Stock Vector Bomb Calorimeter Has A Heat Capacity The heat capacity of the bomb calorimeter is 2.36 kj/ °c. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Four critical parts. Bomb Calorimeter Has A Heat Capacity.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Bomb Calorimeter Has A Heat Capacity Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. Four critical parts are needed in every bomb calorimeter. Calculate the amount of heat released during the combustion. Bomb Calorimeter Has A Heat Capacity.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 Bomb Calorimeter Has A Heat Capacity Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). The heat capacity of the bomb calorimeter is 2.36 kj/ °c. Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. Observation of the temperature rise caused by. Bomb Calorimeter Has A Heat Capacity.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Has A Heat Capacity Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. Four critical parts are needed in every bomb calorimeter. Then use equation 5.6.21 to determine. Bomb Calorimeter Has A Heat Capacity.
From www.numerade.com
SOLVED A bomb calorimeter has a heat capacity of 675 J/PC and contains Bomb Calorimeter Has A Heat Capacity Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the calorimeter which is later. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) +. Bomb Calorimeter Has A Heat Capacity.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Bomb Calorimeter Has A Heat Capacity Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter. Bomb Calorimeter Has A Heat Capacity.
From www.jove.com
Thermochemistry Constant Volume Calorimetry JoVE Book Bomb Calorimeter Has A Heat Capacity Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. The heat capacity of the bomb calorimeter is 2.36 kj/ °c.. Bomb Calorimeter Has A Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Bomb Calorimeter Has A Heat Capacity Four critical parts are needed in every bomb calorimeter. Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. The heat capacity of the. Bomb Calorimeter Has A Heat Capacity.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Bomb Calorimeter Has A Heat Capacity Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the calorimeter which is later. Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)). Bomb Calorimeter Has A Heat Capacity.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Bomb Calorimeter Has A Heat Capacity Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. In chemistry,. Bomb Calorimeter Has A Heat Capacity.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Bomb Calorimeter Has A Heat Capacity Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the calorimeter which is later. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Four critical parts are needed in every bomb calorimeter. 2c 4 h. Bomb Calorimeter Has A Heat Capacity.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Bomb Calorimeter Has A Heat Capacity Four critical parts are needed in every bomb calorimeter. Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h. Bomb Calorimeter Has A Heat Capacity.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter Has A Heat Capacity Four critical parts are needed in every bomb calorimeter. Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. In chemistry,. Bomb Calorimeter Has A Heat Capacity.
From ar.inspiredpencil.com
Bomb Calorimeter Equation Bomb Calorimeter Has A Heat Capacity 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. Observation of the temperature rise caused by this amount of heat enables. Bomb Calorimeter Has A Heat Capacity.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 Bomb Calorimeter Has A Heat Capacity 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. Four critical parts are needed in every bomb calorimeter. Calculate the amount of heat released during the combustion of glucose by multiplying the heat capacity of the bomb by the temperature change. Observation of the temperature rise. Bomb Calorimeter Has A Heat Capacity.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Bomb Calorimeter Has A Heat Capacity Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. Four critical parts are needed in every bomb calorimeter. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. 2c 4. Bomb Calorimeter Has A Heat Capacity.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download Bomb Calorimeter Has A Heat Capacity Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity. Bomb Calorimeter Has A Heat Capacity.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Bomb Calorimeter Has A Heat Capacity Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the calorimeter which is later. Constant volume calorimetry, also know as bomb calorimetry, is used to. Bomb Calorimeter Has A Heat Capacity.
From www.tf.uni-kiel.de
Heat and heat capacity Bomb Calorimeter Has A Heat Capacity In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Observation of the temperature rise caused by this amount of heat enables us to determine the heat capacity of the calorimeter which is later. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. Four critical parts are needed in every bomb. Bomb Calorimeter Has A Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimeter Has A Heat Capacity In chemistry, the changes of heat of a reaction can be measured at fixed pressure or volume. Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by. Four critical parts are needed in every bomb calorimeter. Constant volume calorimetry, also know as bomb calorimetry, is. Bomb Calorimeter Has A Heat Capacity.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Has A Heat Capacity 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) because the combustion of butane. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a. Bomb Calorimeter Has A Heat Capacity.