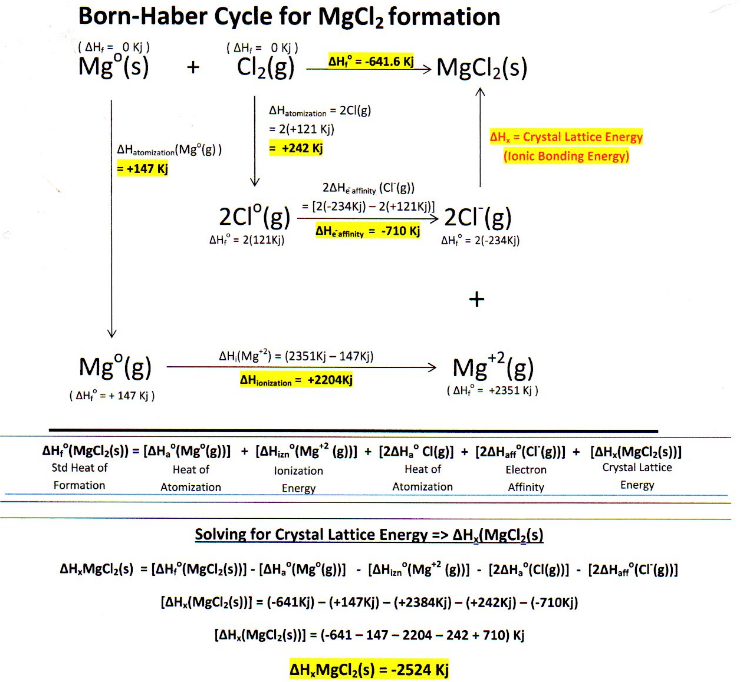
Magnesium Chloride Ionic Formula . 2 negative charges to balance the 2. The magnesium chloride has to be electrically neutral overall. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. To balance the charges you would need 2 chloride ions for every magnesium ion: Working out an ionic formula. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻).
from www.cbsetuts.com
Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. 2 negative charges to balance the 2. Working out an ionic formula. The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻).
What is the chemical formula of magnesium chloride? CBSE Tuts
Magnesium Chloride Ionic Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions for every magnesium ion: Working out an ionic formula. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. 2 negative charges to balance the 2. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻).
From www.elektramagnesium.com.au
Ionic Magnesium Chloride Hexahydrate or Chelated Magnesium? Elektra Magnesium Chloride Ionic Formula To balance the charges you would need 2 chloride ions for every magnesium ion: The magnesium chloride has to be electrically neutral overall. Working out an ionic formula. 2 negative charges to balance the 2. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium. Magnesium Chloride Ionic Formula.
From www.toppr.com
How is magnesium chloride formed by the transfer of electrons? Why does Magnesium Chloride Ionic Formula Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Working out an ionic formula. The magnesium chloride has to be electrically neutral overall. 2 negative charges to balance the 2. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride consists of magnesium cations (mg²⁺). Magnesium Chloride Ionic Formula.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Chloride Ionic Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions for every magnesium ion: Working out an ionic formula. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. 2 negative charges. Magnesium Chloride Ionic Formula.
From proper-cooking.info
Mgcl2 Lewis Structure Magnesium Chloride Ionic Formula To balance the charges you would need 2 chloride ions for every magnesium ion: 2 negative charges to balance the 2. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Working out an ionic formula. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. The magnesium chloride has to. Magnesium Chloride Ionic Formula.
From techiescientist.com
Is MgCl2 Ionic or Covalent? Techiescientist Magnesium Chloride Ionic Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 2 negative charges to balance the 2. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Working out an ionic formula. The magnesium chloride has to. Magnesium Chloride Ionic Formula.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Magnesium Chloride Ionic Formula The magnesium chloride has to be electrically neutral overall. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Working out an ionic formula. To balance the charges you would need 2 chloride ions for every magnesium ion: 2 negative charges to balance the 2. Magnesium chloride consists of magnesium cations (mg²⁺). Magnesium Chloride Ionic Formula.
From www.youtube.com
How to draw dot and cross diagram of magnesium chloride ionic compound Magnesium Chloride Ionic Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 2 negative charges to balance the 2. Working out an ionic formula. The magnesium chloride has to be electrically neutral overall. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. To balance the charges you would need 2 chloride ions. Magnesium Chloride Ionic Formula.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Magnesium Chloride Ionic Formula The magnesium chloride has to be electrically neutral overall. Working out an ionic formula. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 2 negative charges to balance the 2. To balance the charges you would need 2 chloride ions. Magnesium Chloride Ionic Formula.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Magnesium Chloride Ionic Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Working out an ionic formula. The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions for every magnesium ion: 2 negative charges. Magnesium Chloride Ionic Formula.
From diagramedia.blogspot.com
Electron Dot Diagram For Magnesium Chloride Diagram Media Magnesium Chloride Ionic Formula Working out an ionic formula. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 2 negative charges to balance the 2. The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions. Magnesium Chloride Ionic Formula.
From pnghut.com
Lewis Structure Chlorine Atom Chemistry Chloride Magnesium Magnesium Chloride Ionic Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Working out an ionic formula. 2 negative charges to balance the 2. To balance the charges you would need 2 chloride ions for every magnesium ion: The magnesium chloride has to. Magnesium Chloride Ionic Formula.
From ar.inspiredpencil.com
Ionic Bonding Mgcl2 Magnesium Chloride Ionic Formula Working out an ionic formula. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 2 negative charges to balance the 2. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. The magnesium chloride has to. Magnesium Chloride Ionic Formula.
From brainly.in
Write the formation of magnesium chloride (MgCl,) with the help of Magnesium Chloride Ionic Formula Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. To balance the charges you would need 2 chloride ions for every magnesium ion: 2 negative charges to balance the 2. The magnesium chloride has to be electrically neutral overall. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Working. Magnesium Chloride Ionic Formula.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium Chloride Ionic Formula Working out an ionic formula. The magnesium chloride has to be electrically neutral overall. 2 negative charges to balance the 2. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Magnesium chloride consists of magnesium cations (mg²⁺). Magnesium Chloride Ionic Formula.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Chloride Ionic Formula The magnesium chloride has to be electrically neutral overall. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. 2 negative charges to balance the 2. Working out an ionic formula. To balance the charges you would need 2 chloride ions. Magnesium Chloride Ionic Formula.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Magnesium Chloride Ionic Formula The magnesium chloride has to be electrically neutral overall. 2 negative charges to balance the 2. Working out an ionic formula. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium. Magnesium Chloride Ionic Formula.
From favpng.com
Lewis Structure Magnesium Chloride Electron Diagram, PNG, 1024x1024px Magnesium Chloride Ionic Formula 2 negative charges to balance the 2. The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Working. Magnesium Chloride Ionic Formula.
From www.slideserve.com
PPT CHAPTER 3 METALS AND NON METALS PowerPoint Presentation ID598959 Magnesium Chloride Ionic Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Working out an ionic formula. The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions for every magnesium ion: 2 negative charges. Magnesium Chloride Ionic Formula.
From www.science-revision.co.uk
Introduction to ionic bonding Magnesium Chloride Ionic Formula The magnesium chloride has to be electrically neutral overall. 2 negative charges to balance the 2. Working out an ionic formula. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. To balance the charges you would need 2 chloride ions. Magnesium Chloride Ionic Formula.
From inci.guide
Magnesium Chloride Ingredient INCIGuide Magnesium Chloride Ionic Formula Working out an ionic formula. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). The magnesium chloride has to be electrically neutral overall. 2 negative charges. Magnesium Chloride Ionic Formula.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Chloride Ionic Formula Working out an ionic formula. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. 2 negative charges to balance the 2. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). The magnesium chloride has to. Magnesium Chloride Ionic Formula.
From ar.inspiredpencil.com
Magnesium Chloride Structure Magnesium Chloride Ionic Formula The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions for every magnesium ion: Working out an ionic formula. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. 2 negative charges to balance the 2. Magnesium chloride consists of magnesium cations (mg²⁺). Magnesium Chloride Ionic Formula.
From www.slideserve.com
PPT Chemical Equations & Reactions PowerPoint Presentation, free Magnesium Chloride Ionic Formula Working out an ionic formula. The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions for every magnesium ion: 2 negative charges to balance the 2. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Magnesium chloride consists of magnesium cations (mg²⁺). Magnesium Chloride Ionic Formula.
From selfdirectedce.com
How to Write the Net Ionic Equation for Mg + CuCl2 = MgCl2 + Cu Magnesium Chloride Ionic Formula Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 2 negative charges to balance the 2. The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions for every magnesium ion: Working. Magnesium Chloride Ionic Formula.
From www.youtube.com
Is MgCl2 (Magnesium chloride) Ionic or Covalent? YouTube Magnesium Chloride Ionic Formula The magnesium chloride has to be electrically neutral overall. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). To balance the charges you would need 2 chloride ions for every magnesium ion: Working out an ionic formula. 2 negative charges. Magnesium Chloride Ionic Formula.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Magnesium Chloride Ionic Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). To balance the charges you would need 2 chloride ions for every magnesium ion: The magnesium chloride has to be electrically neutral overall. 2 negative charges to balance the 2. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Working. Magnesium Chloride Ionic Formula.
From www.slideshare.net
Chem matters ch6_ionic_bond Magnesium Chloride Ionic Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). The magnesium chloride has to be electrically neutral overall. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. 2 negative charges to balance the 2. To balance the charges you would need 2 chloride ions for every magnesium ion: Working. Magnesium Chloride Ionic Formula.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Magnesium Chloride Ionic Formula 2 negative charges to balance the 2. Working out an ionic formula. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). The magnesium chloride has to be electrically neutral overall. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium. Magnesium Chloride Ionic Formula.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Chloride Ionic Formula Working out an ionic formula. 2 negative charges to balance the 2. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. The magnesium chloride has to. Magnesium Chloride Ionic Formula.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Magnesium Chloride Ionic Formula Working out an ionic formula. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 2 negative charges to balance the 2. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. The magnesium chloride has to. Magnesium Chloride Ionic Formula.
From www.knowledgeboat.com
Chapter 2 Chemical Bonding Selina Solutions Concise Chemistry Class Magnesium Chloride Ionic Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Working out an ionic formula. 2 negative charges to balance the 2. To balance the charges you would need 2 chloride ions for every magnesium ion: The magnesium chloride has to. Magnesium Chloride Ionic Formula.
From schematicsetwall.z14.web.core.windows.net
Correct Electron Dot Diagram For Magnesium Magnesium Chloride Ionic Formula Working out an ionic formula. The magnesium chloride has to be electrically neutral overall. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 2 negative charges to balance the 2. To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium. Magnesium Chloride Ionic Formula.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Magnesium Chloride Ionic Formula 2 negative charges to balance the 2. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. To balance the charges you would need 2 chloride ions for every magnesium ion: The magnesium chloride has to be electrically neutral overall. Working out an ionic formula. Magnesium chloride consists of magnesium cations (mg²⁺). Magnesium Chloride Ionic Formula.
From www.alamy.com
3D image of Magnesium chloride skeletal formula molecular chemical Magnesium Chloride Ionic Formula To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 2 negative charges to balance the 2. Working out an ionic formula. The magnesium chloride has to be electrically neutral overall. Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium. Magnesium Chloride Ionic Formula.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Magnesium Chloride Ionic Formula The magnesium chloride has to be electrically neutral overall. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). To balance the charges you would need 2 chloride ions for every magnesium ion: Magnesium chloride is a chemical compound with the formula mgcl2, consisting of one magnesium and two chloride ions. Working out an ionic formula. 2 negative charges. Magnesium Chloride Ionic Formula.