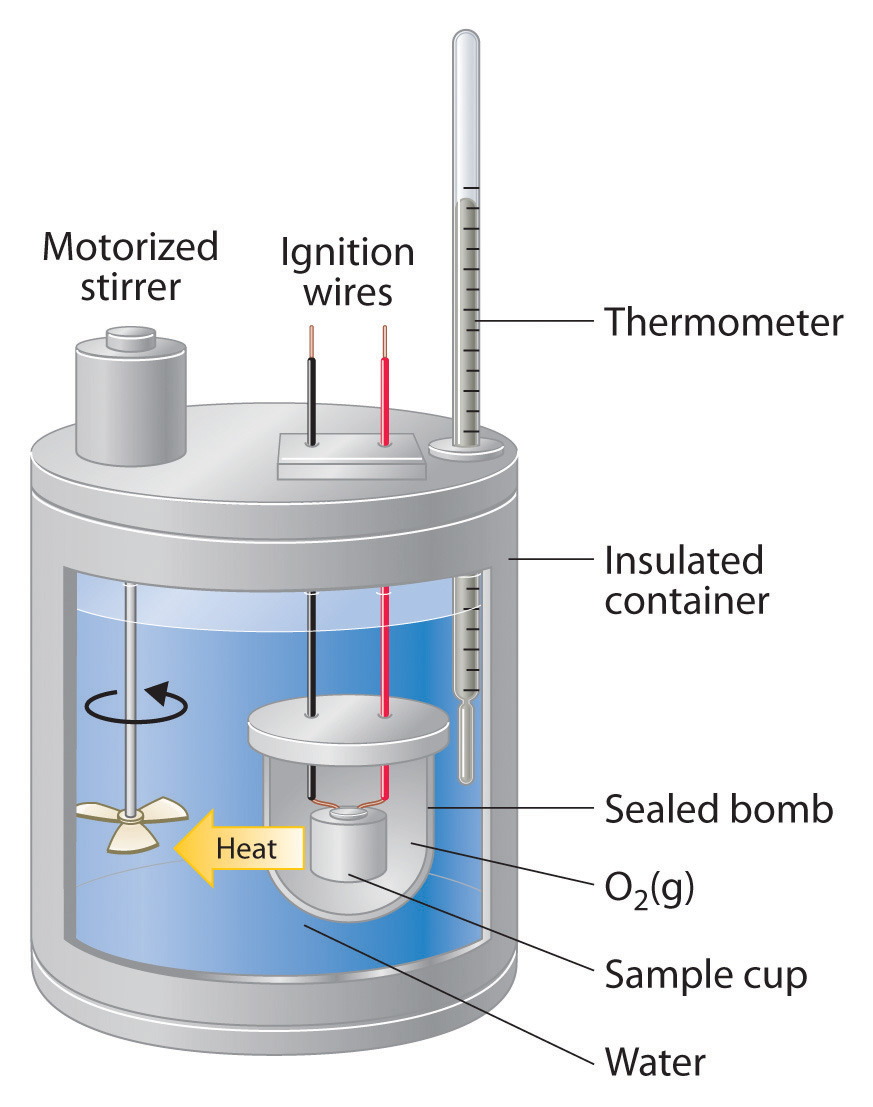
Calorimeter Structure Definition . Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic. For example, when an exothermic.
from chemwiki.ucdavis.edu
For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. For example, when an exothermic.
Chapter 9.6 Calorimetry Chemwiki
Calorimeter Structure Definition Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The process of measuring this heat is called calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. For example, when an exothermic. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Structure Definition For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials.. Calorimeter Structure Definition.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimeter Structure Definition Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic. For example, when an exothermic. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. A calorimeter. Calorimeter Structure Definition.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an. Calorimeter Structure Definition.
From www.researchgate.net
Schematic sketch of a bomb calorimeter Download Scientific Diagram Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical. Calorimeter Structure Definition.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Structure Definition The process of measuring this heat is called calorimetry. For example, when an exothermic reaction occurs in solution in a. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change.. Calorimeter Structure Definition.
From www.jove.com
Thermochemistry Constant Volume Calorimetry JoVE Book Calorimeter Structure Definition Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used. Calorimeter Structure Definition.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. For example, when an. Calorimeter Structure Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Structure Definition Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A. Calorimeter Structure Definition.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter Structure Definition A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Calorimeter Structure Definition.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called calorimetry. A calorimeter measures the mass of liquid and the temperature change of the. Calorimeter Structure Definition.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter Structure Definition Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimeter Structure Definition.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. The process of measuring. Calorimeter Structure Definition.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is. Calorimeter Structure Definition.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Structure Definition For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. For example, when an exothermic reaction occurs in solution in. Calorimeter Structure Definition.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimeter Structure Definition For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Calorimeter, device for measuring the heat developed during a mechanical, electrical,. Calorimeter Structure Definition.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in. Calorimeter Structure Definition.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Structure Definition The process of measuring this heat is called calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A. Calorimeter Structure Definition.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Calorimeter Structure Definition A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. For example, when an exothermic.. Calorimeter Structure Definition.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the. Calorimeter Structure Definition.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter measures the mass of liquid and the temperature change of. Calorimeter Structure Definition.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Calorimeter Structure Definition A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount. Calorimeter Structure Definition.
From library.achievingthedream.org
Calorimetry Introductory Chemistry Lecture & Lab Calorimeter Structure Definition Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in. Calorimeter Structure Definition.
From www.slideserve.com
PPT a “ Calorimeter ” PowerPoint Presentation, free download ID7050684 Calorimeter Structure Definition A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost. Calorimeter Structure Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Structure Definition Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the amount. Calorimeter Structure Definition.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of. Calorimeter Structure Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials.. Calorimeter Structure Definition.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter measures the mass of liquid and the temperature change of the liquid. Calorimeter Structure Definition.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter measures the mass of liquid and. Calorimeter Structure Definition.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimeter Structure Definition Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. A calorimeter is a. Calorimeter Structure Definition.
From www.researchgate.net
Schematic representation of calorimeter system US4 at SKINR. Download Calorimeter Structure Definition Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in. Calorimeter Structure Definition.
From kaffee.50webs.com
Lab Calorimetry Calorimeter Structure Definition A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. For example, when an. Calorimeter Structure Definition.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. For example, when an. Calorimeter Structure Definition.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Structure Definition.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Structure Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter measures the. Calorimeter Structure Definition.
From www.slideserve.com
PPT An introduction to calorimeters for particle physics PowerPoint Calorimeter Structure Definition For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter measures the mass of liquid and the temperature change of. Calorimeter Structure Definition.