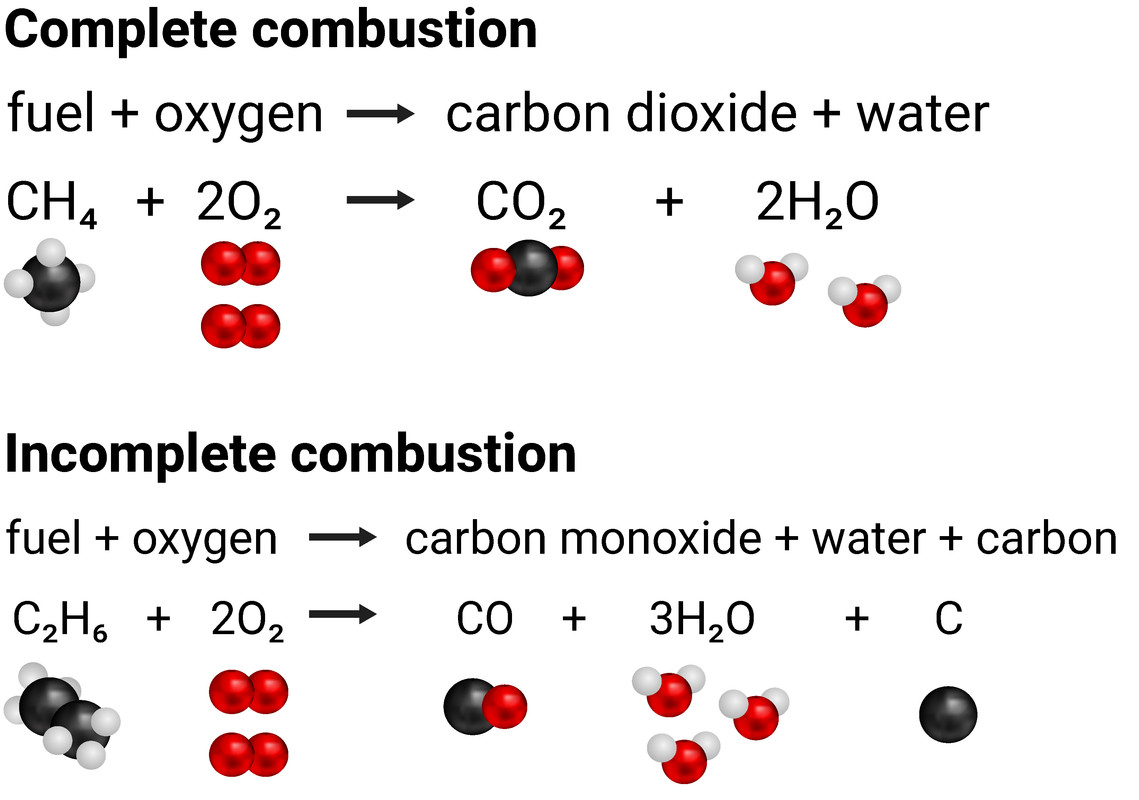
Fuel Gas Chemical Reaction . Combustion is considered an exergonic or exothermic chemical reaction. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. Recognize a reaction as a combustion reaction. This combustion zone is usually called the flame front. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The chemical reaction in such flames occurs within a narrow zone several micrometres thick.
from revisechemistry.uk
Combustion is considered an exergonic or exothermic chemical reaction. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. This combustion zone is usually called the flame front. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Recognize a reaction as a combustion reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
Carbon Compounds as Fuels and Feedstock AQA C7 revisechemistry.uk
Fuel Gas Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. This combustion zone is usually called the flame front. Combustion is considered an exergonic or exothermic chemical reaction. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. Complete and balance chemical equations for combustion reactions. Recognize a reaction as a combustion reaction. The chemical reaction in such flames occurs within a narrow zone several micrometres thick.
From www.teachoo.com
Chemical Reaction Definition, Types and Examples Class 10 Science Fuel Gas Chemical Reaction This combustion zone is usually called the flame front. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Recognize a reaction as a combustion reaction. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form. Fuel Gas Chemical Reaction.
From sciencenotes.org
Combustion Reaction Definition and Examples Fuel Gas Chemical Reaction This combustion zone is usually called the flame front. Combustion is considered an exergonic or exothermic chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in. Fuel Gas Chemical Reaction.
From www.youtube.com
Type of Reaction for CO + O2 = CO2 YouTube Fuel Gas Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is considered an exergonic or exothermic chemical reaction. This combustion zone is usually called the flame front. Complete and balance chemical equations for combustion reactions. Combustion is a chemical reaction that occurs between a fuel and. Fuel Gas Chemical Reaction.
From www.syngaschem.com
Synthesis Gas Chemistry and Synthetic Fuels Syngaschem BV Fuel Gas Chemical Reaction Complete and balance chemical equations for combustion reactions. This combustion zone is usually called the flame front. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. Recognize a reaction as a combustion reaction. Combustion is considered an exergonic or exothermic chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen. Fuel Gas Chemical Reaction.
From www.numerade.com
⏩SOLVEDThe combustion of gasoline produces carbon dioxide and… Numerade Fuel Gas Chemical Reaction Complete and balance chemical equations for combustion reactions. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. This combustion zone is usually called the flame front. Recognize a reaction as a combustion reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and. Fuel Gas Chemical Reaction.
From www.thoughtco.com
An Introduction to Combustion (Burning) Reactions Fuel Gas Chemical Reaction Combustion is considered an exergonic or exothermic chemical reaction. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. This combustion zone is usually called the flame front. A combustion reaction is a reaction in which a substance reacts. Fuel Gas Chemical Reaction.
From revisechemistry.uk
Carbon Compounds as Fuels and Feedstock AQA C7 revisechemistry.uk Fuel Gas Chemical Reaction Complete and balance chemical equations for combustion reactions. Recognize a reaction as a combustion reaction. Combustion is considered an exergonic or exothermic chemical reaction. This combustion zone is usually called the flame front. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. A combustion. Fuel Gas Chemical Reaction.
From www.chegg.com
Solved Hydrogen Fuel Cell Fuel cells are electrochemical Fuel Gas Chemical Reaction Combustion is considered an exergonic or exothermic chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction. Fuel Gas Chemical Reaction.
From maxim-kwise.blogspot.com
The Process by Which a Gas Changes Into a Liquid Fuel Gas Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Recognize a reaction as a combustion reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The chemical reaction in such flames occurs. Fuel Gas Chemical Reaction.
From gavynyouthbooth.blogspot.com
Describe What Happens in a Combustion Reaction Fuel Gas Chemical Reaction Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light. Fuel Gas Chemical Reaction.
From www.alamy.com
Chemical reaction indicators infographics. Chemical changes Fuel Gas Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. The chemical reaction in such flames occurs within a narrow zone several. Fuel Gas Chemical Reaction.
From www.compoundchem.com
The Chemistry of Petrol & The Tetraethyl Lead Story Compound Interest Fuel Gas Chemical Reaction Complete and balance chemical equations for combustion reactions. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. Recognize a reaction as a combustion reaction. Combustion is considered an exergonic or exothermic chemical reaction. The chemical reaction in such flames occurs within a narrow zone. Fuel Gas Chemical Reaction.
From www.youtube.com
Chemistry Lesson Gas Evolution Reactions YouTube Fuel Gas Chemical Reaction This combustion zone is usually called the flame front. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Recognize a reaction as a combustion reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light. Fuel Gas Chemical Reaction.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Fuel Gas Chemical Reaction Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. Recognize a reaction as a combustion reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is considered an exergonic. Fuel Gas Chemical Reaction.
From www.grc.nasa.gov
Combustion Fuel Gas Chemical Reaction Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. Combustion is considered an exergonic or exothermic chemical reaction. Recognize a reaction as a combustion reaction. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. A combustion reaction is a. Fuel Gas Chemical Reaction.
From melscience.com
Interaction of methane with oxygen combustion reaction MEL Chemistry Fuel Gas Chemical Reaction This combustion zone is usually called the flame front. Combustion is considered an exergonic or exothermic chemical reaction. Complete and balance chemical equations for combustion reactions. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of. Fuel Gas Chemical Reaction.
From www.shalom-education.com
Fuels and Combustion Shalom Education Fuel Gas Chemical Reaction This combustion zone is usually called the flame front. Recognize a reaction as a combustion reaction. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The chemical reaction in such flames occurs within a narrow zone several micrometres. Fuel Gas Chemical Reaction.
From www.youtube.com
Balanced Equation for the Combustion of Methane (CH4) YouTube Fuel Gas Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.. Fuel Gas Chemical Reaction.
From sciencetallis.weebly.com
7. Organic Chemistry THOMAS TALLIS SCIENCE Fuel Gas Chemical Reaction Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. This combustion zone is usually called the flame front. Recognize a reaction as a combustion reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form. Fuel Gas Chemical Reaction.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Fuel Gas Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.. Fuel Gas Chemical Reaction.
From shotprofessional22.gitlab.io
Peerless Gas Formation Chemical Reaction 1st Puc Physics Textbook Pdf Fuel Gas Chemical Reaction Combustion is considered an exergonic or exothermic chemical reaction. This combustion zone is usually called the flame front. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Recognize a reaction as a combustion reaction. A combustion reaction is a reaction in which a substance reacts with. Fuel Gas Chemical Reaction.
From studylib.net
COMBUSTION of PROPANE Fuel Gas Chemical Reaction Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. Combustion is considered an exergonic or exothermic chemical reaction. This combustion zone is usually called the flame front. Recognize a reaction. Fuel Gas Chemical Reaction.
From www.scribd.com
Water Gas Shift Reaction and Reactor Modeling For Fuel Cell Fuel Gas Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. Combustion is considered an exergonic or exothermic chemical reaction. Recognize a reaction. Fuel Gas Chemical Reaction.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Fuel Gas Chemical Reaction The chemical reaction in such flames occurs within a narrow zone several micrometres thick. Combustion is considered an exergonic or exothermic chemical reaction. This combustion zone is usually called the flame front. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of. Fuel Gas Chemical Reaction.
From www.teachoo.com
Case Based MCQ Chemistry in Automobiles For an internal combustion Fuel Gas Chemical Reaction Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. Combustion is considered an exergonic or exothermic chemical reaction. Recognize a reaction as a combustion reaction. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. A combustion reaction is a. Fuel Gas Chemical Reaction.
From www.vectorstock.com
Molten carbonate fuel cells process Royalty Free Vector Fuel Gas Chemical Reaction Combustion is considered an exergonic or exothermic chemical reaction. Complete and balance chemical equations for combustion reactions. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. This combustion zone is usually called the flame front. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of. Fuel Gas Chemical Reaction.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Gas Chemical Reaction Combustion is considered an exergonic or exothermic chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Recognize a reaction as a. Fuel Gas Chemical Reaction.
From www.youtube.com
Evidence of Chemical Change Production of a Gas YouTube Fuel Gas Chemical Reaction Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Complete and balance chemical equations for combustion reactions. The chemical reaction in. Fuel Gas Chemical Reaction.
From www.dreamstime.com
Fuel Combustion Process. Different Types of Fuel. Vector Illustration Fuel Gas Chemical Reaction Recognize a reaction as a combustion reaction. This combustion zone is usually called the flame front. Combustion is considered an exergonic or exothermic chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is a chemical reaction that occurs between a fuel and an. Fuel Gas Chemical Reaction.
From www.chemistrylearner.com
Gas Evolution Reaction Definition and Examples Fuel Gas Chemical Reaction Recognize a reaction as a combustion reaction. This combustion zone is usually called the flame front. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form. Fuel Gas Chemical Reaction.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Fuel Gas Chemical Reaction Recognize a reaction as a combustion reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. This combustion zone is usually. Fuel Gas Chemical Reaction.
From www.shutterstock.com
Gas Reaction Equation Gas Evolution Reaction Stock Vector (Royalty Free Fuel Gas Chemical Reaction The chemical reaction in such flames occurs within a narrow zone several micrometres thick. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. Combustion is considered an exergonic or exothermic chemical reaction. This combustion zone is usually called the flame front. Complete and balance. Fuel Gas Chemical Reaction.
From www.researchgate.net
Oxidation reactions in fuel cells (cathodic and anodic half reactions Fuel Gas Chemical Reaction This combustion zone is usually called the flame front. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. Combustion is a chemical reaction that. Fuel Gas Chemical Reaction.
From www.youtube.com
Video Demonstration Chemical Reactions Involving Gas Formation Part 2 Fuel Gas Chemical Reaction Recognize a reaction as a combustion reaction. Combustion is considered an exergonic or exothermic chemical reaction. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in which a substance reacts with oxygen. Fuel Gas Chemical Reaction.
From www.wisegeek.com
What is Fuel Combustion? (with pictures) Fuel Gas Chemical Reaction This combustion zone is usually called the flame front. Complete and balance chemical equations for combustion reactions. The chemical reaction in such flames occurs within a narrow zone several micrometres thick. Recognize a reaction as a combustion reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and. Fuel Gas Chemical Reaction.