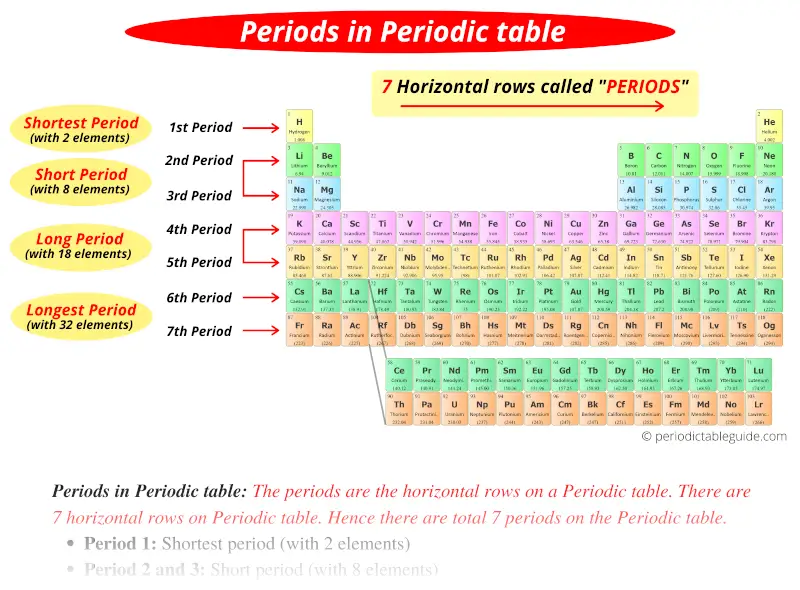
How Are Periods Arranged On The Periodic Table . A new period begins when a new principal energy level begins. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. There are seven periods on the periodic table. There are seven periods in the periodic table, with each one beginning at the far left. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. The elements astatine (at at) and radon (rn rn) are both in period 6. Elements in the same period all have the same electron ground state energy level. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties. A period is a horizontal row of elements on the periodic table.
from periodictableguide.com
The elements astatine (at at) and radon (rn rn) are both in period 6. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. A period is a horizontal row of elements on the periodic table. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties. There are seven periods on the periodic table. There are seven periods in the periodic table, with each one beginning at the far left. A new period begins when a new principal energy level begins. Elements in the same period all have the same electron ground state energy level. Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number.
Periods in Periodic table Explained! (With 17 Period Names)
How Are Periods Arranged On The Periodic Table As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. There are seven periods in the periodic table, with each one beginning at the far left. A new period begins when a new principal energy level begins. The elements astatine (at at) and radon (rn rn) are both in period 6. Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties. There are seven periods on the periodic table. Elements in the same period all have the same electron ground state energy level. A period is a horizontal row of elements on the periodic table. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3.
From www.americanelements.com
Periodic Table of the Elements Toolbox AMERICAN ELEMENTS How Are Periods Arranged On The Periodic Table Elements in the same period all have the same electron ground state energy level. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have. How Are Periods Arranged On The Periodic Table.
From chemistrytalk.org
How to Read the Periodic Table Groups & Periods ChemTalk How Are Periods Arranged On The Periodic Table For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. The elements astatine (at at) and radon (rn rn) are both in period 6. Elements in the same period all have the same electron ground state energy level. A new period begins when a new principal energy level begins. A period is a horizontal. How Are Periods Arranged On The Periodic Table.
From studiousguy.com
Introduction to Periodic Table StudiousGuy How Are Periods Arranged On The Periodic Table As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. There are seven periods in the periodic table, with each one beginning at the far left. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column. How Are Periods Arranged On The Periodic Table.
From period-faqs.com
How Is The Modern Periodic Table Arranged How Are Periods Arranged On The Periodic Table For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties. Periodic table, in chemistry, the organized array of all the chemical elements in order of. How Are Periods Arranged On The Periodic Table.
From www.amnh.org
How to Read the Periodic Table AMNH How Are Periods Arranged On The Periodic Table There are seven periods in the periodic table, with each one beginning at the far left. The elements astatine (at at) and radon (rn rn) are both in period 6. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. When the elements are thus arranged, there is a recurring pattern. How Are Periods Arranged On The Periodic Table.
From utedzz.blogspot.com
Periodic Table With Different Groups Labeled Periodic Table Timeline How Are Periods Arranged On The Periodic Table There are seven periods on the periodic table. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. The elements astatine (at at) and radon (rn rn) are both in period 6. A new period begins when a new principal energy level begins. A period is a horizontal row of elements on the periodic. How Are Periods Arranged On The Periodic Table.
From sciencetrends.com
How Is The Periodic Table Organized And Arranged? Science Trends How Are Periods Arranged On The Periodic Table A period is a horizontal row of elements on the periodic table. Elements in the same period all have the same electron ground state energy level. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. There are seven periods in the periodic table, with each one beginning at the far left. When the. How Are Periods Arranged On The Periodic Table.
From www.livescience.com
Periodic Table of Elements Live Science How Are Periods Arranged On The Periodic Table There are seven periods on the periodic table. Elements in the same period all have the same electron ground state energy level. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in. How Are Periods Arranged On The Periodic Table.
From mammothmemory.net
Elements of periodic table are arranged into groups/periods How Are Periods Arranged On The Periodic Table Elements in the same period all have the same electron ground state energy level. There are seven periods in the periodic table, with each one beginning at the far left. There are seven periods on the periodic table. A period is a horizontal row of elements on the periodic table. The elements astatine (at at) and radon (rn rn) are. How Are Periods Arranged On The Periodic Table.
From glossary.periodni.com
Periodic table of the elements Chemistry Dictionary & Glossary How Are Periods Arranged On The Periodic Table There are seven periods in the periodic table, with each one beginning at the far left. Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. The elements astatine (at at) and radon (rn rn) are both in period 6. For example, the elements sodium (na na) and magnesium (mg mg) are. How Are Periods Arranged On The Periodic Table.
From studylib.net
The Periodic Table How Are Periods Arranged On The Periodic Table Elements in the same period all have the same electron ground state energy level. There are seven periods in the periodic table, with each one beginning at the far left. There are seven periods on the periodic table. The elements astatine (at at) and radon (rn rn) are both in period 6. A new period begins when a new principal. How Are Periods Arranged On The Periodic Table.
From www.thoughtco.com
Properties of Periodic Table of Element Groups How Are Periods Arranged On The Periodic Table Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. The elements astatine (at at) and radon (rn rn) are both in period 6. There are seven periods in the periodic table, with. How Are Periods Arranged On The Periodic Table.
From courses.lumenlearning.com
Reading The Periodic Table of Elements Biology (Early Release) How Are Periods Arranged On The Periodic Table A period is a horizontal row of elements on the periodic table. There are seven periods on the periodic table. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. The elements astatine (at at) and radon (rn rn) are both in period 6. There are seven periods in the periodic table, with each. How Are Periods Arranged On The Periodic Table.
From www.aplustopper.com
What is the periodic table of the elements? A Plus Topper How Are Periods Arranged On The Periodic Table There are seven periods in the periodic table, with each one beginning at the far left. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. Elements in the same period all have the same electron ground state energy level. A period is a horizontal row of elements on the periodic table. The elements. How Are Periods Arranged On The Periodic Table.
From www.allaboutcircuits.com
Table (portrait view) Periodic Table of the Elements Electronics How Are Periods Arranged On The Periodic Table A period is a horizontal row of elements on the periodic table. The elements astatine (at at) and radon (rn rn) are both in period 6. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties. A new period begins when. How Are Periods Arranged On The Periodic Table.
From mungfali.com
Periodic Table Groups And Periods Labeled How Are Periods Arranged On The Periodic Table Elements in the same period all have the same electron ground state energy level. Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. For example, the elements sodium (na na) and magnesium. How Are Periods Arranged On The Periodic Table.
From www.priyamstudycentre.com
Periodic Table Elements, Definition, Groups, Periods, Blocks How Are Periods Arranged On The Periodic Table A new period begins when a new principal energy level begins. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. There are seven periods. How Are Periods Arranged On The Periodic Table.
From www.slideserve.com
PPT Families of the Periodic Table PowerPoint Presentation, free How Are Periods Arranged On The Periodic Table As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. The elements astatine (at at) and radon (rn rn) are both in period 6. Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. There are seven periods on the periodic table. A. How Are Periods Arranged On The Periodic Table.
From utedzz.blogspot.com
Periodic Table Of Elements Labeled Periodic Table Timeline How Are Periods Arranged On The Periodic Table There are seven periods on the periodic table. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. A period is a horizontal row of elements on the periodic table. There are seven periods in the periodic table, with each one beginning at the far left. The elements astatine (at at). How Are Periods Arranged On The Periodic Table.
From www.britannica.com
periodic table Definition & Groups Britannica How Are Periods Arranged On The Periodic Table When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties. The elements astatine (at at) and radon (rn rn) are both in period 6. There are seven periods on the periodic table. For example, the elements sodium (na na) and magnesium. How Are Periods Arranged On The Periodic Table.
From www.slideserve.com
PPT The Periodic Table and the Elements PowerPoint Presentation, free How Are Periods Arranged On The Periodic Table Elements in the same period all have the same electron ground state energy level. There are seven periods on the periodic table. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. A new period begins when a new principal energy level begins. A period is a horizontal row of elements. How Are Periods Arranged On The Periodic Table.
From periodictableguide.com
How are the Elements Arranged in the Modern Periodic Table? How Are Periods Arranged On The Periodic Table A new period begins when a new principal energy level begins. There are seven periods on the periodic table. A period is a horizontal row of elements on the periodic table. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties.. How Are Periods Arranged On The Periodic Table.
From iperiodictable.com
Free Labeled Periodic Table of Elements with Name [PDF & PNG How Are Periods Arranged On The Periodic Table A new period begins when a new principal energy level begins. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. There are seven periods on the periodic table. The elements astatine (at at) and radon (rn rn) are both in period 6. Elements in the same period all have the same electron ground. How Are Periods Arranged On The Periodic Table.
From mavink.com
Periodic Table Elements Explained How Are Periods Arranged On The Periodic Table Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. The elements astatine (at at) and radon (rn rn) are both in period 6. A new period begins when a new principal energy level begins. A. How Are Periods Arranged On The Periodic Table.
From commons.wikimedia.org
FilePeriodic Table Chart.png Wikimedia Commons How Are Periods Arranged On The Periodic Table Elements in the same period all have the same electron ground state energy level. A new period begins when a new principal energy level begins. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties. A period is a horizontal row. How Are Periods Arranged On The Periodic Table.
From www.visionlearning.com
The Periodic Table of Elements Chemistry Visionlearning How Are Periods Arranged On The Periodic Table Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. A new period begins when a new principal energy level begins. There are seven periods in the periodic table, with each one beginning at the far left. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’. How Are Periods Arranged On The Periodic Table.
From newtondesk.com
Periodic Table Groups And Periods Of Elements Chemistry How Are Periods Arranged On The Periodic Table Elements in the same period all have the same electron ground state energy level. There are seven periods in the periodic table, with each one beginning at the far left. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. There are seven periods on the periodic table. When the elements. How Are Periods Arranged On The Periodic Table.
From periodictableguide.com
How Is Periodic Table Arranged (By Atomic Mass or Number?) How Are Periods Arranged On The Periodic Table Elements in the same period all have the same electron ground state energy level. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. For example, the elements sodium (na na) and magnesium. How Are Periods Arranged On The Periodic Table.
From periodictableguide.com
Periods in Periodic table Explained! (With 17 Period Names) How Are Periods Arranged On The Periodic Table A new period begins when a new principal energy level begins. The elements astatine (at at) and radon (rn rn) are both in period 6. There are seven periods in the periodic table, with each one beginning at the far left. There are seven periods on the periodic table. Elements in the same period all have the same electron ground. How Are Periods Arranged On The Periodic Table.
From mungfali.com
Periodic Table Groups And Periods Labeled How Are Periods Arranged On The Periodic Table A period is a horizontal row of elements on the periodic table. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. When the elements are thus arranged, there is a recurring pattern. How Are Periods Arranged On The Periodic Table.
From www.webelements.com
The periodic table of the elements by Elements How Are Periods Arranged On The Periodic Table When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties. A period is a horizontal row of elements on the periodic table. There are seven periods in the periodic table, with each one beginning at the far left. As you move. How Are Periods Arranged On The Periodic Table.
From periodictable.me
Labeled Periodic Table of Elements with Name Dynamic Periodic Table How Are Periods Arranged On The Periodic Table A period is a horizontal row of elements on the periodic table. A new period begins when a new principal energy level begins. For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. There are. How Are Periods Arranged On The Periodic Table.
From periodictableguide.com
How are the Elements Arranged in the Modern Periodic Table? How Are Periods Arranged On The Periodic Table There are seven periods on the periodic table. Elements in the same period all have the same electron ground state energy level. Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. The elements astatine (at at) and radon (rn rn) are both in period 6. When the elements are thus arranged,. How Are Periods Arranged On The Periodic Table.
From sciencenotes.org
Periodic Table Groups and Periods How Are Periods Arranged On The Periodic Table For example, the elements sodium (na na) and magnesium (mg mg) are both in period 3. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties. A new period begins when a new principal energy level begins. There are seven periods. How Are Periods Arranged On The Periodic Table.
From www.periodictableprintable.com
Periodic Table Of Elements With Group Numbers 2023 Periodic Table How Are Periods Arranged On The Periodic Table Elements in the same period all have the same electron ground state energy level. There are seven periods on the periodic table. There are seven periods in the periodic table, with each one beginning at the far left. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in. How Are Periods Arranged On The Periodic Table.