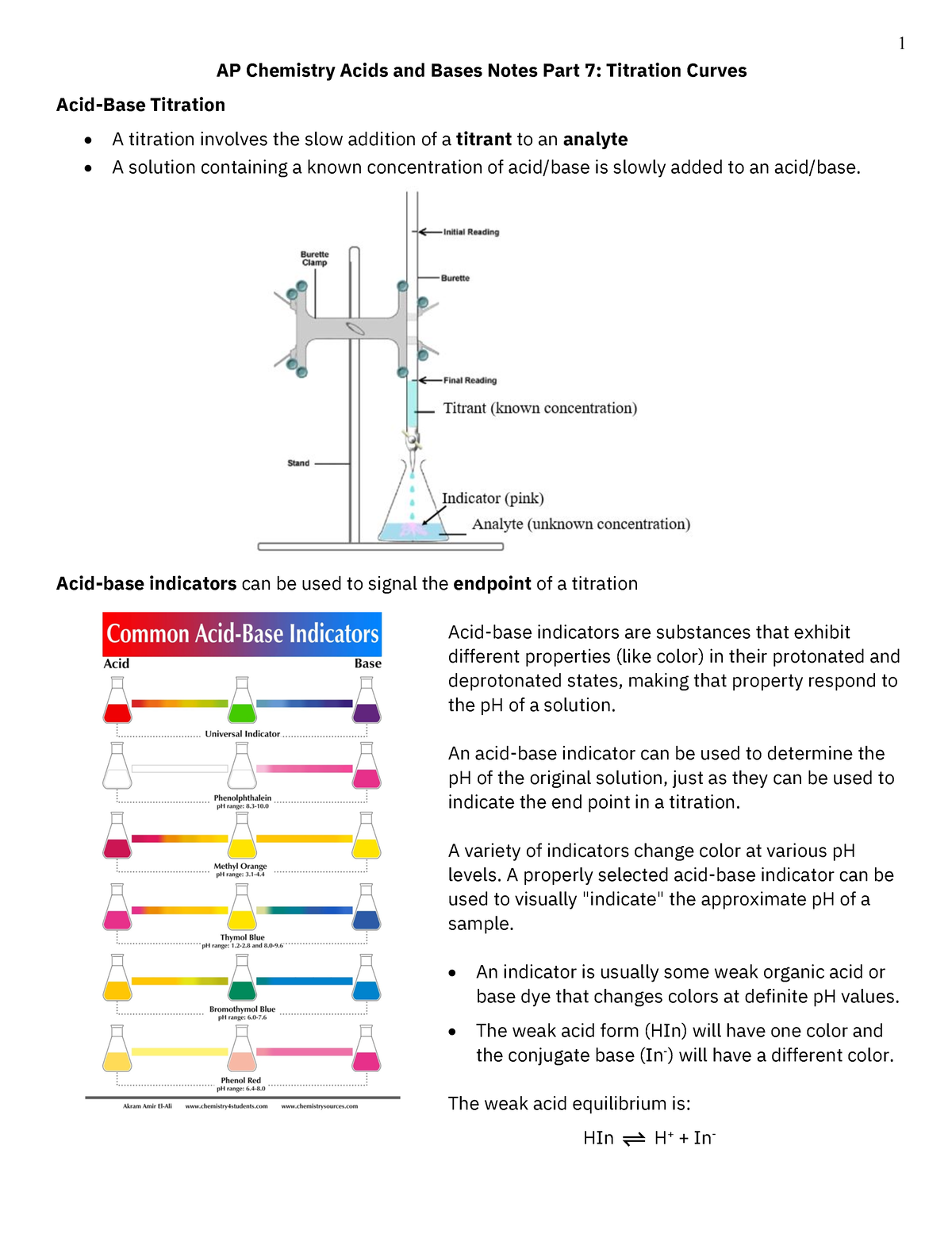
Acid-Base Titration General Chemistry . In an indicator based titration you add another. A titration can be performed with almost any chemical reaction for which. Titration is a common laboratory technique for determining the concentration of a solute. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. The reagent (titrant) is the solution with a known molarity that will react with the analyte. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. It is based on the neutralization reaction, where.
from www.studocu.com
Titration is a common laboratory technique for determining the concentration of a solute. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. A titration can be performed with almost any chemical reaction for which. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. It is based on the neutralization reaction, where.
AP Chemistry Acids and Bases NotesTitration Curves AP Chemistry
Acid-Base Titration General Chemistry The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. There are two basic types of acid base titrations, indicator and potentiometric. Titration is a common laboratory technique for determining the concentration of a solute. The analyte (titrand) is the solution with an unknown molarity. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. It is based on the neutralization reaction, where. A titration can be performed with almost any chemical reaction for which. The reagent (titrant) is the solution with a known molarity that will react with the analyte.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Acid-Base Titration General Chemistry Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. It is based on the neutralization reaction, where. The analyte (titrand) is the solution with an unknown molarity. Titration is a common laboratory technique for determining the concentration of a solute. The reagent (titrant) is the solution with a known molarity that will react. Acid-Base Titration General Chemistry.
From www.numerade.com
SOLVED CHEM 101 GENERAL CHEMISTRY Lab 7 AcidBase Titration (A Acid-Base Titration General Chemistry The analyte (titrand) is the solution with an unknown molarity. Titration is a common laboratory technique for determining the concentration of a solute. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. The reagent (titrant) is the solution with a known molarity that will react with the analyte.. Acid-Base Titration General Chemistry.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Acid-Base Titration General Chemistry A titration can be performed with almost any chemical reaction for which. The analyte (titrand) is the solution with an unknown molarity. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. Titration is a common laboratory technique for determining the concentration of a solute. In an indicator based. Acid-Base Titration General Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Acid-Base Titration General Chemistry The reagent (titrant) is the solution with a known molarity that will react with the analyte. It is based on the neutralization reaction, where. In an indicator based titration you add another. Titration is a common laboratory technique for determining the concentration of a solute. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand). Acid-Base Titration General Chemistry.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Acid-Base Titration General Chemistry Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. There are two basic types of acid base titrations, indicator and potentiometric. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. In an indicator based titration you add another. A titration can. Acid-Base Titration General Chemistry.
From mungfali.com
Acid Base Titration Calculation Acid-Base Titration General Chemistry It is based on the neutralization reaction, where. Titration is a common laboratory technique for determining the concentration of a solute. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. In an. Acid-Base Titration General Chemistry.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Acid-Base Titration General Chemistry In an indicator based titration you add another. It is based on the neutralization reaction, where. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Titration is a common laboratory technique for determining the concentration of a solute. The analyte (titrand) is the solution with an unknown molarity. Calculate the molarity of a. Acid-Base Titration General Chemistry.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Acid-Base Titration General Chemistry Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Titration is a common laboratory technique for determining the concentration of a solute. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. There are two basic types of acid base titrations, indicator. Acid-Base Titration General Chemistry.
From www.docsity.com
Acid Base Titrations General Chemistry Lab CHEM 1045 Docsity Acid-Base Titration General Chemistry A titration can be performed with almost any chemical reaction for which. In an indicator based titration you add another. Titration is a common laboratory technique for determining the concentration of a solute. There are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where. Calculate the molarity of a monoprotic acid. Acid-Base Titration General Chemistry.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Acid-Base Titration General Chemistry There are two basic types of acid base titrations, indicator and potentiometric. The reagent (titrant) is the solution with a known molarity that will react with the analyte. A titration can be performed with almost any chemical reaction for which. It is based on the neutralization reaction, where. In an indicator based titration you add another. Titration is a common. Acid-Base Titration General Chemistry.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Acid-Base Titration General Chemistry There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. Titration is a common laboratory technique for determining the concentration of a solute. The reagent (titrant) is the solution with a known molarity that will react with the analyte. A titration can be performed with almost any chemical. Acid-Base Titration General Chemistry.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Acid-Base Titration General Chemistry A titration can be performed with almost any chemical reaction for which. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Titration is a common laboratory technique for determining the concentration of a solute. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and. Acid-Base Titration General Chemistry.
From www.youtube.com
Acids and Bases, Titrations, and Neutralization Reactions YouTube Acid-Base Titration General Chemistry The analyte (titrand) is the solution with an unknown molarity. There are two basic types of acid base titrations, indicator and potentiometric. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. A. Acid-Base Titration General Chemistry.
From www.studocu.com
AP Chemistry Acids and Bases NotesTitration Curves AP Chemistry Acid-Base Titration General Chemistry It is based on the neutralization reaction, where. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. There are two basic types of acid base titrations, indicator and potentiometric. Titration is a common laboratory technique for determining the concentration of a solute. Performing. Acid-Base Titration General Chemistry.
From www.studocu.com
Lecture Notes 9 + Experiment 9 ACIDBASE TITRATION Experiment 9 Acid-Base Titration General Chemistry In an indicator based titration you add another. The reagent (titrant) is the solution with a known molarity that will react with the analyte. It is based on the neutralization reaction, where. There are two basic types of acid base titrations, indicator and potentiometric. Titration is a common laboratory technique for determining the concentration of a solute. A titration can. Acid-Base Titration General Chemistry.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid-Base Titration General Chemistry Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Titration is a common laboratory technique for determining the concentration of a solute. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. The analyte (titrand) is the solution with an unknown molarity. It. Acid-Base Titration General Chemistry.
From www.youtube.com
Acid Base Neutralization Reactions & Net Ionic Equations Chemistry Acid-Base Titration General Chemistry Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. It is based on the neutralization reaction, where. Titration is a common laboratory technique for determining the concentration of a solute. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. A. Acid-Base Titration General Chemistry.
From studylib.net
Acid/Base Titration Lab Acid-Base Titration General Chemistry A titration can be performed with almost any chemical reaction for which. It is based on the neutralization reaction, where. In an indicator based titration you add another. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. Performing chemical reactions quantitatively to determine. Acid-Base Titration General Chemistry.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Acid-Base Titration General Chemistry The reagent (titrant) is the solution with a known molarity that will react with the analyte. There are two basic types of acid base titrations, indicator and potentiometric. A titration can be performed with almost any chemical reaction for which. It is based on the neutralization reaction, where. In an indicator based titration you add another. The analyte (titrand) is. Acid-Base Titration General Chemistry.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Acid-Base Titration General Chemistry In an indicator based titration you add another. The reagent (titrant) is the solution with a known molarity that will react with the analyte. It is based on the neutralization reaction, where. The analyte (titrand) is the solution with an unknown molarity. Titration is a common laboratory technique for determining the concentration of a solute. There are two basic types. Acid-Base Titration General Chemistry.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Acid-Base Titration General Chemistry A titration can be performed with almost any chemical reaction for which. In an indicator based titration you add another. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Titration is a common laboratory technique for determining the concentration of a solute. There are two basic types of acid base titrations, indicator and. Acid-Base Titration General Chemistry.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry Acid-Base Titration General Chemistry It is based on the neutralization reaction, where. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand). Acid-Base Titration General Chemistry.
From www.docsity.com
AcidBase Titrations General Chemistry Lab CHEM 1045 Docsity Acid-Base Titration General Chemistry It is based on the neutralization reaction, where. The reagent (titrant) is the solution with a known molarity that will react with the analyte. There are two basic types of acid base titrations, indicator and potentiometric. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. Performing chemical reactions. Acid-Base Titration General Chemistry.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Acid-Base Titration General Chemistry Titration is a common laboratory technique for determining the concentration of a solute. The analyte (titrand) is the solution with an unknown molarity. A titration can be performed with almost any chemical reaction for which. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. The reagent (titrant) is. Acid-Base Titration General Chemistry.
From www.studocu.com
CHEM 1108 PreLab Weak AcidStrong Base Titration General Chemistry Acid-Base Titration General Chemistry A titration can be performed with almost any chemical reaction for which. There are two basic types of acid base titrations, indicator and potentiometric. Titration is a common laboratory technique for determining the concentration of a solute. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. In an indicator based titration you add. Acid-Base Titration General Chemistry.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Acid-Base Titration General Chemistry It is based on the neutralization reaction, where. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. A titration can be performed with almost any chemical reaction for which. The reagent (titrant) is the solution with a known molarity. Acid-Base Titration General Chemistry.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Acid-Base Titration General Chemistry There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. Titration is a common laboratory technique for determining the concentration of a solute. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Calculate the molarity of a monoprotic acid ha whose titration endpoint. Acid-Base Titration General Chemistry.
From www.youtube.com
Acid Base Titration Lecture 3 Chemistry Practicals Fsc 1st Year Acid-Base Titration General Chemistry In an indicator based titration you add another. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Titration is a common laboratory technique for determining the concentration. Acid-Base Titration General Chemistry.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Acid-Base Titration General Chemistry The reagent (titrant) is the solution with a known molarity that will react with the analyte. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. In an indicator based titration you add another. The analyte (titrand) is the solution with an unknown molarity. There are two basic types. Acid-Base Titration General Chemistry.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Acid-Base Titration General Chemistry Titration is a common laboratory technique for determining the concentration of a solute. In an indicator based titration you add another. A titration can be performed with almost any chemical reaction for which. The analyte (titrand) is the solution with an unknown molarity. There are two basic types of acid base titrations, indicator and potentiometric. The reagent (titrant) is the. Acid-Base Titration General Chemistry.
From www.youtube.com
17.3a Strong Acid Strong Base Titrations pH Calculations General Acid-Base Titration General Chemistry The reagent (titrant) is the solution with a known molarity that will react with the analyte. Titration is a common laboratory technique for determining the concentration of a solute. A titration can be performed with almost any chemical reaction for which. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and. Acid-Base Titration General Chemistry.
From saylordotorg.github.io
AcidBase Titrations Acid-Base Titration General Chemistry It is based on the neutralization reaction, where. A titration can be performed with almost any chemical reaction for which. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. The reagent (titrant) is. Acid-Base Titration General Chemistry.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Acid-Base Titration General Chemistry There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. It is based on the neutralization reaction, where. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. The reagent (titrant) is the solution with a known molarity that will react with. Acid-Base Titration General Chemistry.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Acid-Base Titration General Chemistry Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another. A titration can be performed with almost any chemical reaction for which. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v. Acid-Base Titration General Chemistry.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Acid-Base Titration General Chemistry Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. In an indicator based titration you add another. It is based on the neutralization reaction, where. Calculate the molarity of a monoprotic acid ha whose titration endpoint occurs after v ml of strong base of a given. The reagent (titrant) is the solution with. Acid-Base Titration General Chemistry.