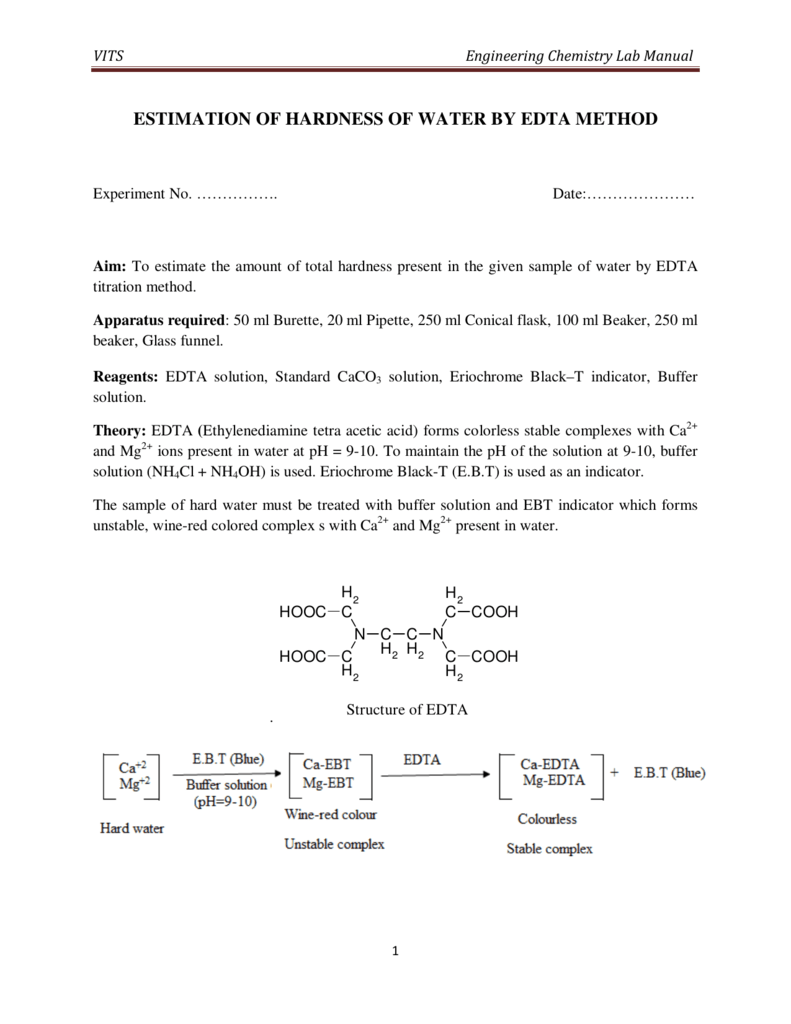
Indicator Used In Hardness Test Of Water . Calcium and magnesium salts dissolved in water cause water hardness. The ions involved in water hardness, i.e. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. Hardness in water can be determined quickly by titration and the use of color indicators. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. The value reported for hardness includes all divalent ions in a water sample. Complexometric titration is one of the best ways of measuring total water hardness. At ph around 10 edta easily reacts with both calcium. Three indicators are commonly used in edta titration, eriochrome. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible.
from studylib.net
Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. Three indicators are commonly used in edta titration, eriochrome. Hardness in water can be determined quickly by titration and the use of color indicators. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Calcium and magnesium salts dissolved in water cause water hardness. The ions involved in water hardness, i.e. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. The value reported for hardness includes all divalent ions in a water sample. Complexometric titration is one of the best ways of measuring total water hardness.
ESTIMATION OF HARDNESS OF WATER BY EDTA METHOD
Indicator Used In Hardness Test Of Water Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. The ions involved in water hardness, i.e. The value reported for hardness includes all divalent ions in a water sample. Complexometric titration is one of the best ways of measuring total water hardness. Calcium and magnesium salts dissolved in water cause water hardness. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. Three indicators are commonly used in edta titration, eriochrome. At ph around 10 edta easily reacts with both calcium. Hardness in water can be determined quickly by titration and the use of color indicators. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. By proper choice of ph, total hardness (ca 2+ and mg 2+) or.
From www.foreverpureplace.com
Hach Hardness 2 Indicator Solution, 4 Oz Indicator Used In Hardness Test Of Water Calcium and magnesium salts dissolved in water cause water hardness. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. At ph around 10 edta easily reacts with both calcium. Hardness in water can be determined quickly by titration and the use of color indicators. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. The. Indicator Used In Hardness Test Of Water.
From tektrak.co.uk
Water Hardness Test Strips Indicator Used In Hardness Test Of Water Calcium and magnesium salts dissolved in water cause water hardness. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. Three indicators are commonly used in edta titration, eriochrome. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Water hardness can be measured using a titration. Indicator Used In Hardness Test Of Water.
From www.taylortechnologies.com
Hardness Indicator Powder Indicator Used In Hardness Test Of Water Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. The value reported for hardness includes all divalent ions in a water sample. The ions involved in water hardness, i.e. Three indicators are commonly used in edta titration, eriochrome.. Indicator Used In Hardness Test Of Water.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube Indicator Used In Hardness Test Of Water By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Calcium and magnesium salts dissolved in water cause water hardness. At ph around 10 edta easily reacts with both calcium. Complexometric titration is one of the best ways of measuring total water hardness. The value reported for hardness includes all divalent ions in a water sample. Hardness. Indicator Used In Hardness Test Of Water.
From www.rakiro.net
Total Hardness Test Kit (500 Test) Indicator Used In Hardness Test Of Water Calcium and magnesium salts dissolved in water cause water hardness. Hardness in water can be determined quickly by titration and the use of color indicators. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. The ions involved in water hardness, i.e. At ph around 10 edta easily reacts with both calcium. The value reported for hardness. Indicator Used In Hardness Test Of Water.
From www.yuzuki.co.in
Rubber Hardness Tester Indicator Used In Hardness Test Of Water Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Three indicators are commonly used in edta titration, eriochrome. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. The value reported for hardness includes all. Indicator Used In Hardness Test Of Water.
From www.honeforest.net
Water Hardness Test Strips HoneForest Indicator Used In Hardness Test Of Water Complexometric titration is one of the best ways of measuring total water hardness. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. Edta and the metallochromic indicators used are involved in complexation reactions. Indicator Used In Hardness Test Of Water.
From www.thepondguy.com
Water Hardness Test Strips Pond Water Test The Pond Guy Indicator Used In Hardness Test Of Water The value reported for hardness includes all divalent ions in a water sample. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. Complexometric titration is one of the best ways of measuring total water hardness. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. At ph. Indicator Used In Hardness Test Of Water.
From www.desertcart.in
Buy Water Hardness Test Kit 250 Tests Best OEM Wholesale price Indicator Used In Hardness Test Of Water At ph around 10 edta easily reacts with both calcium. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Calcium and magnesium salts dissolved in water cause water hardness. The value reported for hardness includes all divalent ions in a water sample. The ions involved in water hardness, i.e. Complexometric titration is one of the best. Indicator Used In Hardness Test Of Water.
From waternitylab.com
Water Hardness Scale GPG, mmol/L, PPM Chart Indicator Used In Hardness Test Of Water At ph around 10 edta easily reacts with both calcium. Calcium and magnesium salts dissolved in water cause water hardness. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. Three indicators are commonly used in edta titration, eriochrome. The ions involved in water hardness, i.e. By proper choice of ph, total hardness (ca 2+ and mg 2+) or.. Indicator Used In Hardness Test Of Water.
From studylib.net
ESTIMATION OF HARDNESS OF WATER BY EDTA METHOD Indicator Used In Hardness Test Of Water Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. Calcium and magnesium salts dissolved in water cause water hardness. Complexometric titration is one of the best ways of measuring total water hardness. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. Hardness. Indicator Used In Hardness Test Of Water.
From www.bestrowaterpurifier.in
How to Check Water Hardness 3 Easy Ways of Water Hardness Test Indicator Used In Hardness Test Of Water Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. Three indicators are commonly used in edta titration, eriochrome. Hardness in water can be determined quickly by titration and the use of color indicators. At ph around 10 edta easily reacts with both calcium. Complexometric titration is one of the. Indicator Used In Hardness Test Of Water.
From ubicaciondepersonas.cdmx.gob.mx
Luxury Water Hardness Test Kit ubicaciondepersonas.cdmx.gob.mx Indicator Used In Hardness Test Of Water At ph around 10 edta easily reacts with both calcium. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. Complexometric titration is one of the best ways of measuring total water hardness. The value reported for hardness includes all divalent ions in a water sample. Hardness in water can be determined quickly by titration and the use. Indicator Used In Hardness Test Of Water.
From www.bwtshop.co.uk
Luxury Water Hardness Test Kit for testing total water hardness in ppm/ml Indicator Used In Hardness Test Of Water Calcium and magnesium salts dissolved in water cause water hardness. The value reported for hardness includes all divalent ions in a water sample. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. At ph around 10 edta easily reacts with both calcium. Hardness in water can be determined quickly by titration and the use of color indicators.. Indicator Used In Hardness Test Of Water.
From www.forestry-suppliers.com
Hach Total Hardness Test Strips Forestry Suppliers, Inc. Indicator Used In Hardness Test Of Water The ions involved in water hardness, i.e. Calcium and magnesium salts dissolved in water cause water hardness. The value reported for hardness includes all divalent ions in a water sample. Hardness in water can be determined quickly by titration and the use of color indicators. Three indicators are commonly used in edta titration, eriochrome. Edta and the metallochromic indicators used. Indicator Used In Hardness Test Of Water.
From www.shopclues.com
Buy Aquasol Total Hardness Test Kit Online Get 11 Off Indicator Used In Hardness Test Of Water By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Three indicators are commonly used in edta titration, eriochrome. Hardness in water can be determined quickly by titration and the use of color indicators. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. At ph around 10 edta easily reacts with both calcium. The. Indicator Used In Hardness Test Of Water.
From www.toolstation.com
Water Hardness Test Kit Toolstation Indicator Used In Hardness Test Of Water By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Calcium and magnesium salts dissolved in water cause water hardness. The ions involved in water hardness, i.e. At ph around 10 edta easily reacts with both calcium. Hardness in water can be determined quickly by titration and the use of color indicators. Three indicators are commonly used. Indicator Used In Hardness Test Of Water.
From www.snapdeal.com
Buy Water Hardness Testing Kit TDS+TEMP Meter 2in1 Portable Pen Type Indicator Used In Hardness Test Of Water Complexometric titration is one of the best ways of measuring total water hardness. The value reported for hardness includes all divalent ions in a water sample. Three indicators are commonly used in edta titration, eriochrome. The ions involved in water hardness, i.e. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. Calcium and magnesium salts dissolved in water. Indicator Used In Hardness Test Of Water.
From www.ebay.com
Digital Water Quality Test Pen EC TDS Tester PH Meter Water Hardness Indicator Used In Hardness Test Of Water Hardness in water can be determined quickly by titration and the use of color indicators. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. The ions involved in water hardness, i.e. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. Complexometric titration is one of the. Indicator Used In Hardness Test Of Water.
From bahrain.desertcart.com
Buy Health Metric Pro Water Hardness Test Kit Quick & Easy Hard Water Indicator Used In Hardness Test Of Water At ph around 10 edta easily reacts with both calcium. Hardness in water can be determined quickly by titration and the use of color indicators. The value reported for hardness includes all divalent ions in a water sample. Complexometric titration is one of the best ways of measuring total water hardness. Calcium and magnesium salts dissolved in water cause water. Indicator Used In Hardness Test Of Water.
From waterguides.org
Best Water Hardness Test Kit Indicator Used In Hardness Test Of Water Complexometric titration is one of the best ways of measuring total water hardness. The value reported for hardness includes all divalent ions in a water sample. Three indicators are commonly used in edta titration, eriochrome. Hardness in water can be determined quickly by titration and the use of color indicators. Calcium and magnesium salts dissolved in water cause water hardness.. Indicator Used In Hardness Test Of Water.
From varify.com
FAQ/Resource Hardness Test Strips Indicator Used In Hardness Test Of Water At ph around 10 edta easily reacts with both calcium. Hardness in water can be determined quickly by titration and the use of color indicators. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. The ions involved in water hardness, i.e. Edta and the. Indicator Used In Hardness Test Of Water.
From waterfilterspot.com
Water Hardness Scale For Water Softener The Ideal Number Indicator Used In Hardness Test Of Water By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. The ions involved in water hardness, i.e. Calcium and magnesium salts dissolved in water cause water hardness. Complexometric titration is one of the best ways of measuring total water hardness. Three indicators are commonly used. Indicator Used In Hardness Test Of Water.
From www.carolina.com
LaMotte® Total Hardness Water Test Kit Carolina Biological Supply Indicator Used In Hardness Test Of Water By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. Calcium and magnesium salts dissolved in water cause water hardness. Three indicators are commonly used in edta titration, eriochrome. Complexometric titration is one of the best ways of measuring total water hardness. Edta and the. Indicator Used In Hardness Test Of Water.
From itechnhealth.com
How Do You Know If You Have Hard Water Indicator Used In Hardness Test Of Water Three indicators are commonly used in edta titration, eriochrome. At ph around 10 edta easily reacts with both calcium. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. The ions involved in water hardness, i.e. Calcium and magnesium salts dissolved in water cause water hardness. Complexometric titration is one. Indicator Used In Hardness Test Of Water.
From www.desertcart.ae
Buy Pack of 10 Aquadur Water Hardness Strips with Colourful Scale for Indicator Used In Hardness Test Of Water Hardness in water can be determined quickly by titration and the use of color indicators. The value reported for hardness includes all divalent ions in a water sample. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. At ph around 10 edta easily reacts with both calcium. Calcium and. Indicator Used In Hardness Test Of Water.
From www.slideshare.net
Lab5 determination of hardness of water Indicator Used In Hardness Test Of Water Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. Hardness in water can be determined quickly by titration and the use of color indicators. Three indicators are commonly used in edta titration, eriochrome. Complexometric titration is one of the best ways of measuring total water hardness.. Indicator Used In Hardness Test Of Water.
From pureaquausa.com
Water Testing Kits PureAquaUSA for Drinking Water testing at home Indicator Used In Hardness Test Of Water By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. Complexometric titration is one of the best ways of measuring total water hardness. At ph around 10 edta easily reacts with both calcium. The value reported for hardness includes all divalent ions in a water sample.. Indicator Used In Hardness Test Of Water.
From exoervuzv.blob.core.windows.net
Water Hardness Test Jura at Gregory Ducan blog Indicator Used In Hardness Test Of Water The ions involved in water hardness, i.e. Three indicators are commonly used in edta titration, eriochrome. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. The value reported for hardness includes all divalent ions in a water sample. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. At ph around 10 edta easily. Indicator Used In Hardness Test Of Water.
From exowhppfg.blob.core.windows.net
Lead Hardness Indicator at Charlotte Chandler blog Indicator Used In Hardness Test Of Water The value reported for hardness includes all divalent ions in a water sample. Complexometric titration is one of the best ways of measuring total water hardness. At ph around 10 edta easily reacts with both calcium. Calcium and magnesium salts dissolved in water cause water hardness. The ions involved in water hardness, i.e. By proper choice of ph, total hardness. Indicator Used In Hardness Test Of Water.
From www.slideserve.com
PPT Water Hardness Determination with EDTA PowerPoint Presentation Indicator Used In Hardness Test Of Water Complexometric titration is one of the best ways of measuring total water hardness. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Three indicators are commonly used in edta titration, eriochrome. Calcium and magnesium salts dissolved. Indicator Used In Hardness Test Of Water.
From www.youtube.com
Water Hardness Test Strips How To Test For Water Hardness? YouTube Indicator Used In Hardness Test Of Water Complexometric titration is one of the best ways of measuring total water hardness. Hardness in water can be determined quickly by titration and the use of color indicators. At ph around 10 edta easily reacts with both calcium. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. By proper choice of ph, total hardness (ca 2+ and. Indicator Used In Hardness Test Of Water.
From www.pharmaqualification.com
Hardness Testing of Water Indicator Used In Hardness Test Of Water Three indicators are commonly used in edta titration, eriochrome. By proper choice of ph, total hardness (ca 2+ and mg 2+) or. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. At ph around 10 edta easily reacts with both calcium. Calcium and magnesium salts dissolved in water cause. Indicator Used In Hardness Test Of Water.
From www.btsa.in
Hardness Testing Bombay Tools Supplying Agency Indicator Used In Hardness Test Of Water The value reported for hardness includes all divalent ions in a water sample. Hardness in water can be determined quickly by titration and the use of color indicators. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid. Edta and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are responsible. Ca2+(aq). Indicator Used In Hardness Test Of Water.
From www.varifytest.com
Water Hardness Test Kit 100 Strips at 0425ppm Indicator Used In Hardness Test Of Water The value reported for hardness includes all divalent ions in a water sample. Complexometric titration is one of the best ways of measuring total water hardness. Ca2+(aq) and mg2+(aq), can be determined by titration with a chelating agent,. Three indicators are commonly used in edta titration, eriochrome. Edta and the metallochromic indicators used are involved in complexation reactions with the. Indicator Used In Hardness Test Of Water.