Titratable Acidity Definition Urine . — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. This is the normal ph. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. Creatinine (pka approx 5.0) may also.
from labpedia.net
titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. This is the normal ph. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka. titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. Creatinine (pka approx 5.0) may also.
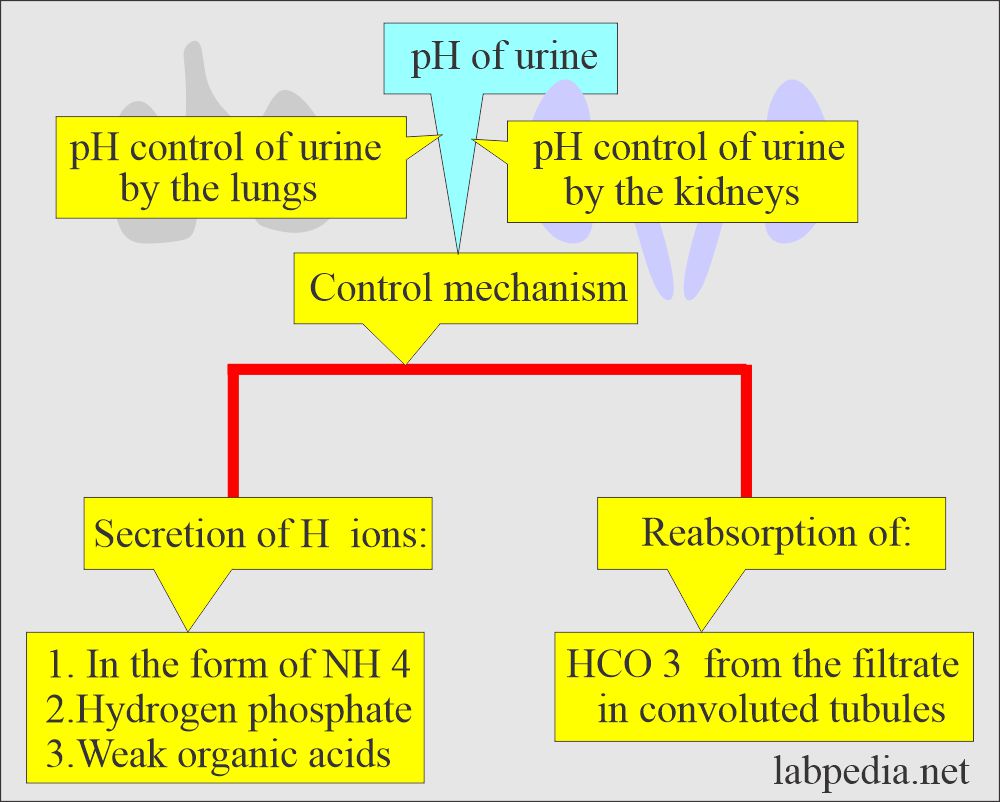
Urine pH and Its Significance
Titratable Acidity Definition Urine — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. Creatinine (pka approx 5.0) may also. This is the normal ph. — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration.
From www.researchgate.net
Titratable acidity of urine ( ) and feeds ( ) for a 24h period from Titratable Acidity Definition Urine — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by. Titratable Acidity Definition Urine.
From labpedia.net
Urine pH and Its Significance Titratable Acidity Definition Urine This is the normal ph. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. Creatinine (pka approx 5.0) may also. — titratable acidity (ta) in urine can. Titratable Acidity Definition Urine.
From www.researchgate.net
Urinary pH, net acid excretion (NAE), titratable acidity (TA), and Titratable Acidity Definition Urine This is the normal ph. Creatinine (pka approx 5.0) may also. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. titratable acid is defined as the. Titratable Acidity Definition Urine.
From www.youtube.com
Titratable Acid Handling in the Nephron YouTube Titratable Acidity Definition Urine — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. Creatinine (pka approx 5.0) may also. This is the normal ph. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka. titratable acidity (ta) in urine. Titratable Acidity Definition Urine.
From doctorlib.info
RENAL MECHANISMS IN ACIDBASE BALANCE AcidBase Physiology Titratable Acidity Definition Urine titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. Creatinine (pka approx 5.0) may also. — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. — titratable acidity (ta) in urine can be measured directly. Titratable Acidity Definition Urine.
From www.slideserve.com
PPT Lecture7 RENAL HANDLING OF ACIDBASE BALANCE PowerPoint Titratable Acidity Definition Urine — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by. Titratable Acidity Definition Urine.
From doctorlib.info
Regulation of Renal Acid Secretion Transport of Acids and Bases The Titratable Acidity Definition Urine — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. This is the normal ph. — titratable acidity (ta) in urine can be measured directly. Titratable Acidity Definition Urine.
From present5.com
Examination of Urine Terry Kotrla MS MT ASCP Professor Titratable Acidity Definition Urine titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. titratable acidity represents the h + which is buffered mostly by phosphate which is. Titratable Acidity Definition Urine.
From teletalkbd.com
A Comprehensive Guide to Titratable Acidity Test Kits The Key to Titratable Acidity Definition Urine — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka. Creatinine (pka approx 5.0) may also. titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. — titratable acidity (ta) in urine can be. Titratable Acidity Definition Urine.
From www.researchgate.net
Relationship between urine titratable acidity and components of acid Titratable Acidity Definition Urine — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. Creatinine (pka approx 5.0) may also. titratable acid is defined as the amount of strong base needed to. Titratable Acidity Definition Urine.
From derangedphysiology.com
Renal regulation of acidbase balance Deranged Physiology Titratable Acidity Definition Urine — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka. — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant. Titratable Acidity Definition Urine.
From www.chegg.com
Solved Titratable acid excretion is quantified by measuring Titratable Acidity Definition Urine — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. This is the normal ph. — titratable acidity (ta) in urine can be measured directly or. Titratable Acidity Definition Urine.
From www.mak95.com
AcidBase Titratable Acidity Definition Urine This is the normal ph. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. Creatinine (pka approx 5.0) may also. — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. titratable acidity represents the h + which. Titratable Acidity Definition Urine.
From www.youtube.com
Renal Mechanism For The Regulation of pH Acid Base Balance YouTube Titratable Acidity Definition Urine — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant. Titratable Acidity Definition Urine.
From www.mediscicenter.com
URINE ORGANIC ACID TEST mediscicenter Titratable Acidity Definition Urine — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. Creatinine (pka approx 5.0) may also. — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. titratable acidity (ta) in urine can be measured directly or calculated. Titratable Acidity Definition Urine.
From www.studypool.com
SOLUTION Analysis of macromolecules and titratable acidity of human Titratable Acidity Definition Urine — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka. Creatinine (pka approx 5.0) may also. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. titratable acidity (ta) in urine can be measured directly or calculated from. Titratable Acidity Definition Urine.
From www.researchgate.net
Responses of urinary ammonia and titratable acid excretion to Titratable Acidity Definition Urine This is the normal ph. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. titratable acidity (ta) in urine can be measured directly or calculated from actual and. Titratable Acidity Definition Urine.
From www.researchgate.net
Titratable acidity of urine ( ) and feeds (•) from lambs fed increasing Titratable Acidity Definition Urine titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. Creatinine (pka approx 5.0) may also. — titratable acidity (ta) in urine can be measured. Titratable Acidity Definition Urine.
From slideplayer.com
AcidBase Regulation1 Lecture 8 (9/4/2015) ppt download Titratable Acidity Definition Urine titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back. Titratable Acidity Definition Urine.
From www.researchgate.net
(PDF) Calculation of titratable acidity from urinary stone risk factors Titratable Acidity Definition Urine This is the normal ph. — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. — titratable acidity (ta) in urine can be measured directly or calculated. Titratable Acidity Definition Urine.
From slideplayer.com
Division of Nephrology, Department of Internal Medicine, Wonkwang Univ Titratable Acidity Definition Urine Creatinine (pka approx 5.0) may also. titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. — titratable acidity (ta) in urine can be measured directly or. Titratable Acidity Definition Urine.
From www.researchgate.net
Relative urinary titratable acid and ammonia in adults on a control Titratable Acidity Definition Urine — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka. titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. titratable acidity represents the h + which is buffered mostly by phosphate which is. Titratable Acidity Definition Urine.
From slidetodoc.com
AcidBase and ABG Interpretation Made Simple Aa Gradient Titratable Acidity Definition Urine titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. Creatinine (pka approx 5.0) may also. titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. — other forms of urinary acids were. Titratable Acidity Definition Urine.
From www.researchgate.net
Procedure to determine titratable acidity in a sample by the sensor Titratable Acidity Definition Urine titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. This is the normal ph. — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. titratable acidity represents the h + which is buffered mostly by. Titratable Acidity Definition Urine.
From labmonk.com
Estimation of Titrable Acidity and Ammonia in Urine Sample Labmonk Titratable Acidity Definition Urine This is the normal ph. titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. — titratable acidity (ta) in urine can be. Titratable Acidity Definition Urine.
From www.researchgate.net
Mean Titratable Acidity in the Organic Acids Download Scientific Diagram Titratable Acidity Definition Urine titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. Creatinine (pka approx 5.0) may also. — titratable acidity (ta) in urine can be measured directly or. Titratable Acidity Definition Urine.
From labpedia.net
Acidbase Balance Part 1 A Introduction to the AcidBase Balance Titratable Acidity Definition Urine titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. titratable acid is defined as the amount of strong base needed to titrate the ph of the. Titratable Acidity Definition Urine.
From slidetodoc.com
Renal Physiology 10 AcidBase Balance 2 Buffers System Titratable Acidity Definition Urine Creatinine (pka approx 5.0) may also. This is the normal ph. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. titratable acid is defined as the amount of strong base needed to titrate the ph of the urine back to ph 7.4. — titratable acidity (ta). Titratable Acidity Definition Urine.
From www.slideshare.net
Acid base balance Titratable Acidity Definition Urine — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. This is the normal ph. titratable acid is defined as the amount of strong base needed. Titratable Acidity Definition Urine.
From www.researchgate.net
Responses of urinary ammonia and titratable acid excretion to Titratable Acidity Definition Urine titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka. — other forms of urinary acids were already recognized at that time [40], and. Titratable Acidity Definition Urine.
From www.researchgate.net
Relative urinary titratable acid and ammonia in adults on a control Titratable Acidity Definition Urine — other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant. Titratable Acidity Definition Urine.
From criticalcarecollaborative.com
Renal Physiology Critical Care Collaborative Titratable Acidity Definition Urine — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph,. Titratable Acidity Definition Urine.
From www.researchgate.net
Daily urinary net acid excretion (titratable acid NH 4 HCO 3 ) in Titratable Acidity Definition Urine titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka₂ 6,8 for. — during daily amino acid metabolism, acids (mostly in the form of phosphates, but also sulfate, hippurate and citrate) are. — other forms of urinary acids were already recognized at that time [40], and these. Titratable Acidity Definition Urine.
From www.slideserve.com
PPT The Renal Role in Acid Base Balance PowerPoint Presentation, free Titratable Acidity Definition Urine — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka2. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. titratable acid is defined as the amount of strong base needed to titrate the ph of the urine. Titratable Acidity Definition Urine.
From www.youtube.com
TITRATABLE ACIDITY IN URINE YouTube Titratable Acidity Definition Urine Creatinine (pka approx 5.0) may also. titratable acidity represents the h + which is buffered mostly by phosphate which is present in significant concentration. — titratable acidity (ta) in urine can be measured directly or calculated from actual and reference ph, by using the pka. This is the normal ph. titratable acid is defined as the amount. Titratable Acidity Definition Urine.