Catalysts Ib Chemistry . They do so, by providing an alternative mechanism (route) for the reaction to proceed. The reaction occurs at active sites on the surface of the catalyst. There are two types of catalyst: A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst; A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction. International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions A heterogeneous catalyst is in a different physical state (phase) from the reactants.
from www.mdpi.com
A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst; A heterogeneous catalyst is in a different physical state (phase) from the reactants. There are two types of catalyst: A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction. International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. They do so, by providing an alternative mechanism (route) for the reaction to proceed. A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. The reaction occurs at active sites on the surface of the catalyst.
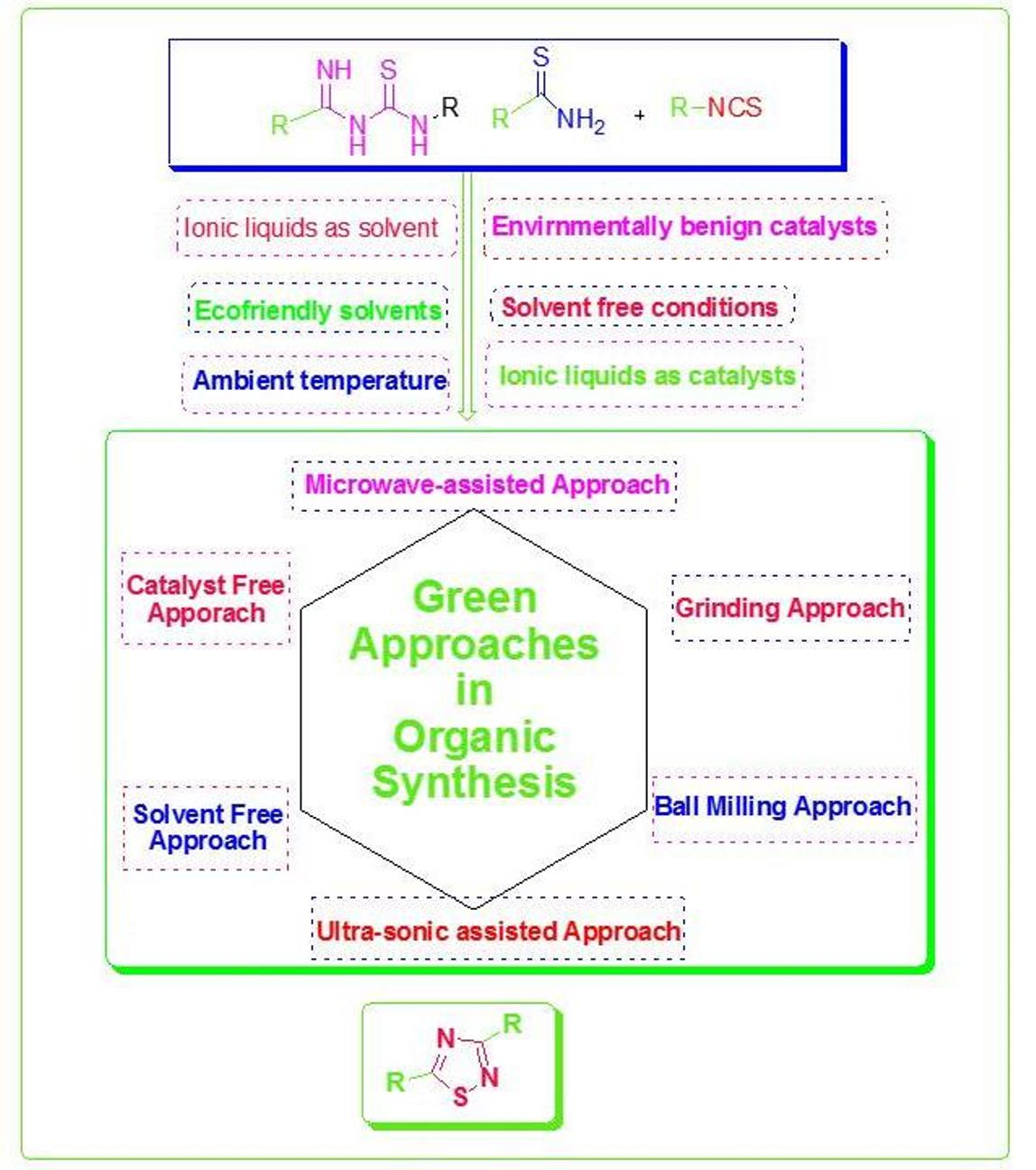
Catalysts Free FullText Green Chemistry in Organic Synthesis
Catalysts Ib Chemistry They do so, by providing an alternative mechanism (route) for the reaction to proceed. A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. There are two types of catalyst: A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst; A heterogeneous catalyst is in a different physical state (phase) from the reactants. Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. The reaction occurs at active sites on the surface of the catalyst. International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions They do so, by providing an alternative mechanism (route) for the reaction to proceed. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction.
From www.slideserve.com
PPT IB Chemistry PowerPoint Presentation, free download ID2738432 Catalysts Ib Chemistry A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction. A heterogeneous catalyst is in a different physical state (phase) from the reactants. A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. There are two types. Catalysts Ib Chemistry.
From shop.sserltd.com
Catalysis SSER Ltd. Shop Catalysts Ib Chemistry There are two types of catalyst: International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. The reaction occurs at active sites on the surface of the catalyst. They do so, by providing an. Catalysts Ib Chemistry.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalysts Ib Chemistry International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions They do so, by providing an alternative mechanism (route) for the reaction to proceed. Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. The reaction occurs at active sites on the. Catalysts Ib Chemistry.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalysts Ib Chemistry A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. A heterogeneous catalyst is in a different physical state (phase) from the reactants. If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst;. Catalysts Ib Chemistry.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts Ib Chemistry The reaction occurs at active sites on the surface of the catalyst. A heterogeneous catalyst is in a different physical state (phase) from the reactants. If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst; There are two types of catalyst: A catalyst is a substance that affects the. Catalysts Ib Chemistry.
From www.nagwa.com
Lesson Catalysts Nagwa Catalysts Ib Chemistry The reaction occurs at active sites on the surface of the catalyst. A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst; A. Catalysts Ib Chemistry.
From www.slideserve.com
PPT IB Chemistry PowerPoint Presentation, free download ID2738432 Catalysts Ib Chemistry A heterogeneous catalyst is in a different physical state (phase) from the reactants. International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction. A catalyst is a substance that increases the. Catalysts Ib Chemistry.
From www.mdpi.com
Catalysts Free FullText Advances in Catalytic of N2O Catalysts Ib Chemistry They do so, by providing an alternative mechanism (route) for the reaction to proceed. A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. The reaction occurs at active sites on the surface of the catalyst. A heterogeneous catalyst is in a different physical state (phase) from the reactants. Catalysts. Catalysts Ib Chemistry.
From www.cademix.org
Applications of Heterogeneous Catalysis in Industry Catalysts Ib Chemistry International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative. Catalysts Ib Chemistry.
From www.youtube.com
Catalysts and Homogeneous and Heterogeneous Catalysis (ALevel IB Catalysts Ib Chemistry A heterogeneous catalyst is in a different physical state (phase) from the reactants. International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction. The reaction occurs at active sites on the. Catalysts Ib Chemistry.
From scitechdaily.com
The Future of Petrochemicals Chemical Looping and CoreShell Catalysts Catalysts Ib Chemistry A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction. A heterogeneous catalyst is in a different physical state (phase) from the reactants. A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of. Catalysts Ib Chemistry.
From www.tes.com
Organic reaction pathways for OCR A Level Chemistry A Teaching Resources Catalysts Ib Chemistry A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. The reaction occurs at active sites on the surface of the catalyst. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative. Catalysts Ib Chemistry.
From www.mdpi.com
Catalysts Free FullText Green Chemistry in Organic Synthesis Catalysts Ib Chemistry The reaction occurs at active sites on the surface of the catalyst. Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. There are two types of catalyst:. Catalysts Ib Chemistry.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalysts Ib Chemistry They do so, by providing an alternative mechanism (route) for the reaction to proceed. A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction.. Catalysts Ib Chemistry.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalysts Ib Chemistry They do so, by providing an alternative mechanism (route) for the reaction to proceed. There are two types of catalyst: Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. If all other conditions stay the same, the equilibrium constant k c is not affected by the presence. Catalysts Ib Chemistry.
From www.chemie.de
Organic Chemistry Reaction Map Catalysts Ib Chemistry A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. They do so, by providing an alternative mechanism (route) for the reaction to proceed. A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction.. Catalysts Ib Chemistry.
From www.linkedin.com
Cell Press on LinkedIn Read the latest issue of Chem Catalysis. Online Catalysts Ib Chemistry There are two types of catalyst: A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. They do so, by providing an alternative mechanism (route) for the reaction to proceed. A heterogeneous catalyst is in a different physical state (phase) from the reactants. A. Catalysts Ib Chemistry.
From www.hanlin.com
IB DP Chemistry SL复习笔记6.1.8 Energy Profiles & Catalysis翰林国际教育 Catalysts Ib Chemistry There are two types of catalyst: A heterogeneous catalyst is in a different physical state (phase) from the reactants. Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and. Catalysts Ib Chemistry.
From www.researchgate.net
Commonly Used Types of Catalysts and Their Range of Use Download Catalysts Ib Chemistry A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. There are two types of catalyst: If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst; A catalyst is a substance that increases. Catalysts Ib Chemistry.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysts Ib Chemistry There are two types of catalyst: A heterogeneous catalyst is in a different physical state (phase) from the reactants. They do so, by providing an alternative mechanism (route) for the reaction to proceed. The reaction occurs at active sites on the surface of the catalyst. International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper. Catalysts Ib Chemistry.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalysts Ib Chemistry Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. A catalyst is a substance that increases the rate of a chemical. Catalysts Ib Chemistry.
From www.masterorganicchemistry.com
The 4 Major Classes of Reactions in Org 1 Master Organic Chemistry Catalysts Ib Chemistry A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction. International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst;. Catalysts Ib Chemistry.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalysts Ib Chemistry A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst; The reaction occurs at active sites on the surface of the catalyst. There. Catalysts Ib Chemistry.
From www.savemyexams.com
Energy Profiles With & Without Catalysts HL IB Chemistry Revision Catalysts Ib Chemistry If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst; International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction.. Catalysts Ib Chemistry.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalysts Ib Chemistry There are two types of catalyst: Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. The reaction occurs at active sites on the surface of the catalyst. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing. Catalysts Ib Chemistry.
From ar.inspiredpencil.com
Catalyst Examples For Kids Catalysts Ib Chemistry If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst; A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions Catalysts play a. Catalysts Ib Chemistry.
From www.youtube.com
Collision Theory and Catalysts [IB Chemistry SL/HL] YouTube Catalysts Ib Chemistry The reaction occurs at active sites on the surface of the catalyst. A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. A heterogeneous. Catalysts Ib Chemistry.
From www.msjchem.com
Topic 6 MSJChem Tutorial videos for IB Chemistry Catalysts Ib Chemistry The reaction occurs at active sites on the surface of the catalyst. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction. Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. A catalyst is. Catalysts Ib Chemistry.
From www.mdpi.com
Catalysts Free FullText Synthesis of Catalytic Precursors Based on Catalysts Ib Chemistry If all other conditions stay the same, the equilibrium constant k c is not affected by the presence of a catalyst; Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. A heterogeneous catalyst is in a different physical state (phase) from the reactants. A catalyst is a. Catalysts Ib Chemistry.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Ib Chemistry A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. If all other conditions stay the same, the equilibrium constant k c is not. Catalysts Ib Chemistry.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts Ib Chemistry A heterogeneous catalyst is in a different physical state (phase) from the reactants. Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. International baccalaureate chemistry web, an interactive ib syllabus with revision notes and worked past paper questions They do so, by providing an alternative mechanism (route). Catalysts Ib Chemistry.
From chemistnotes.com
Acid base catalysis General vs specific Chemistry Notes Catalysts Ib Chemistry A catalyst is a substance that increases the rate of a chemical reaction without undergoing a permanent chemical change itself. Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. A heterogeneous catalyst is in a different physical state (phase) from the reactants. A catalyst is a substance. Catalysts Ib Chemistry.
From chemistry.stackexchange.com
organic chemistry Hydrogenation stereochemistryPd,Pt, Ni catalysts Catalysts Ib Chemistry The reaction occurs at active sites on the surface of the catalyst. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy and providing an alternative reaction. A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the. Catalysts Ib Chemistry.
From www.mdpi.com
Catalysts Free FullText Green Chemistry in Organic Synthesis Catalysts Ib Chemistry There are two types of catalyst: A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. The reaction occurs at active sites on the surface of the catalyst. They do so, by providing an alternative mechanism (route) for the reaction to proceed. If all. Catalysts Ib Chemistry.
From www.slideserve.com
PPT IB Chemistry PowerPoint Presentation, free download ID2738432 Catalysts Ib Chemistry Catalysts play a crucial role in the field of chemistry by increasing the rate of chemical reactions without being consumed in the. A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. International baccalaureate chemistry web, an interactive ib syllabus with revision notes and. Catalysts Ib Chemistry.