Fda Definition Of Diagnostic Device . The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: Manufacturers can find detailed information about complying with the. How does the eu mdr define a medical device? An overview of how the fda regulates in vitro diagnostic products (ivd). (2) the conditions of use for the device, including. (1) the persons for whose use the device is represented or intended; You should read this first.
from v9306.1blu.de
A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. (2) the conditions of use for the device, including. (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: An overview of how the fda regulates in vitro diagnostic products (ivd). (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. You should read this first. (1) the persons for whose use the device is represented or intended; Manufacturers can find detailed information about complying with the. The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. How does the eu mdr define a medical device?
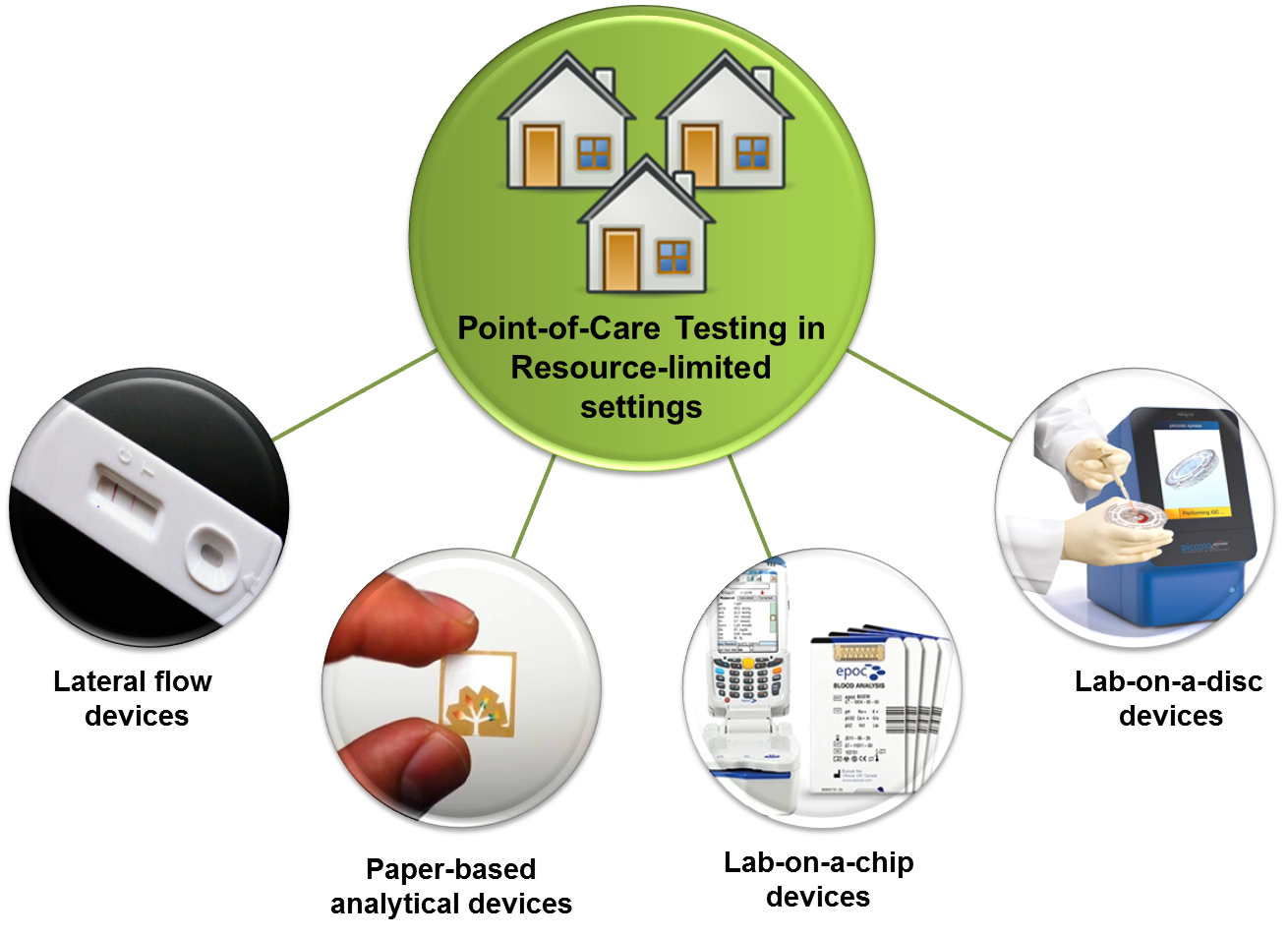
From PointofCare Testing To EHealth Diagnostic Devices
Fda Definition Of Diagnostic Device (2) the conditions of use for the device, including. (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. Manufacturers can find detailed information about complying with the. The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. (1) the persons for whose use the device is represented or intended; How does the eu mdr define a medical device? (2) the conditions of use for the device, including. A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. An overview of how the fda regulates in vitro diagnostic products (ivd). (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: You should read this first.
From www.artfulcompliance.com
00034 Companion Diagnostic Fda Definition Of Diagnostic Device (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: How does the eu mdr define a medical device? The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. (a) in vitro diagnostic products are those reagents, instruments, and systems intended for. Fda Definition Of Diagnostic Device.
From www.slideserve.com
PPT FDA Regulation of In Vitro Diagnostic Tests PowerPoint Fda Definition Of Diagnostic Device (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a.. Fda Definition Of Diagnostic Device.
From rs-ness.com
IVD Classification Under IVDR Definition RS NESS Fda Definition Of Diagnostic Device (2) the conditions of use for the device, including. (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: (1) the persons for whose use the device is represented or intended; How does the eu mdr define a medical device? Manufacturers can find detailed information about complying with the. An overview. Fda Definition Of Diagnostic Device.
From gioixjpns.blob.core.windows.net
Device Definition Fda at Brian Hudgens blog Fda Definition Of Diagnostic Device (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. An overview of how the fda regulates in vitro diagnostic products (ivd). The european medical devices regulation, uses a similar definition of a medical device to. Fda Definition Of Diagnostic Device.
From docslib.org
List of Cleared Or Approved Companion Diagnostic Devices (In Vitro and Fda Definition Of Diagnostic Device An overview of how the fda regulates in vitro diagnostic products (ivd). Manufacturers can find detailed information about complying with the. (1) the persons for whose use the device is represented or intended; How does the eu mdr define a medical device? (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or. Fda Definition Of Diagnostic Device.
From angelanjohnson.com
Medical Devices Angela N Johnson Fda Definition Of Diagnostic Device How does the eu mdr define a medical device? You should read this first. The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. (1) the persons for whose use the device is represented or intended; (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in.. Fda Definition Of Diagnostic Device.
From www.medicaldesignandoutsourcing.com
FDA updates guidance on unclassified devices Medical Design and Fda Definition Of Diagnostic Device (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: Manufacturers can find detailed information about complying with the. (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. How does the eu mdr define a medical device? The european medical devices regulation, uses a similar. Fda Definition Of Diagnostic Device.
From www.researchgate.net
List of FDACleared or Approved Companion Diagnostic Devices (In Vitro Fda Definition Of Diagnostic Device How does the eu mdr define a medical device? (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: An overview of how the fda regulates in vitro diagnostic products (ivd). The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. Manufacturers. Fda Definition Of Diagnostic Device.
From www.slideserve.com
PPT Overview of FDA Regulation of Devices & Diagnostics PowerPoint Fda Definition Of Diagnostic Device A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. How does the eu mdr define a medical device? (2) the conditions of use for. Fda Definition Of Diagnostic Device.
From pr-1733-i-sx-1214-11-ip-35-182-249-18.my.pullpreview.com
Medical Devices & Diagnostics Report Emerging Innovations to Boost Fda Definition Of Diagnostic Device (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: (2) the conditions of use for the device, including. (1) the persons for whose use the device is represented or intended; A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the. Fda Definition Of Diagnostic Device.
From www.researchgate.net
Medical Device Classification System Download Table Fda Definition Of Diagnostic Device An overview of how the fda regulates in vitro diagnostic products (ivd). (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. How does the. Fda Definition Of Diagnostic Device.
From laegemiddelstyrelsen.dk
Classification of in vitro diagnostic medical devices (IVD) Fda Definition Of Diagnostic Device (1) the persons for whose use the device is represented or intended; An overview of how the fda regulates in vitro diagnostic products (ivd). (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: The european. Fda Definition Of Diagnostic Device.
From www.mdpi.com
A Systematic Database Approach to Identify Companion Diagnostic Testing Fda Definition Of Diagnostic Device You should read this first. (1) the persons for whose use the device is represented or intended; A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. An overview of how the fda regulates in vitro diagnostic products (ivd). The european medical devices regulation,. Fda Definition Of Diagnostic Device.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Fda Definition Of Diagnostic Device (2) the conditions of use for the device, including. Manufacturers can find detailed information about complying with the. A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. An. Fda Definition Of Diagnostic Device.
From angelanjohnson.com
DrugDevice Combination Treatments and Diagnostics Roundtable Angela Fda Definition Of Diagnostic Device (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. The european medical devices regulation, uses a similar definition of a medical device to the. Fda Definition Of Diagnostic Device.
From www.slideserve.com
PPT Assessment of biomarker assay and performance when are Fda Definition Of Diagnostic Device (2) the conditions of use for the device, including. (1) the persons for whose use the device is represented or intended; How does the eu mdr define a medical device? An overview of how the fda regulates in vitro diagnostic products (ivd). Manufacturers can find detailed information about complying with the. (k) noninvasive, when applied to a diagnostic device or. Fda Definition Of Diagnostic Device.
From www.proximacro.com
510(k) or PMA Should Your Medical Device Receive FDA Clearance or FDA Fda Definition Of Diagnostic Device (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. An overview of how the fda regulates in vitro diagnostic products (ivd). How does the eu mdr define a medical device? (2) the conditions of use for the device, including. Manufacturers can find detailed information about complying with the. You should read this first. (1). Fda Definition Of Diagnostic Device.
From www.researchgate.net
Overview of the process of in vitro diagnostic (IVD) test development Fda Definition Of Diagnostic Device You should read this first. (2) the conditions of use for the device, including. Manufacturers can find detailed information about complying with the. An overview of how the fda regulates in vitro diagnostic products (ivd). The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. How does the eu mdr define. Fda Definition Of Diagnostic Device.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Fda Definition Of Diagnostic Device The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. How does the eu mdr define a medical device? (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. You should read this first. (k) noninvasive, when applied to a diagnostic device or procedure, means one. Fda Definition Of Diagnostic Device.
From pubs.acs.org
From PointofCare Testing to eHealth Diagnostic Devices (eDiagnostics Fda Definition Of Diagnostic Device (2) the conditions of use for the device, including. (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. (1) the persons for whose use the device is represented or intended; How does the eu mdr define a medical device? A companion diagnostic is a medical device, often an in vitro device, which provides information. Fda Definition Of Diagnostic Device.
From www.slideserve.com
PPT FDA Regulation of In Vitro Diagnostic Tests PowerPoint Fda Definition Of Diagnostic Device (2) the conditions of use for the device, including. Manufacturers can find detailed information about complying with the. (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. How. Fda Definition Of Diagnostic Device.
From www.slideserve.com
PPT Overview of FDA Regulation of Devices & Diagnostics PowerPoint Fda Definition Of Diagnostic Device The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not. Fda Definition Of Diagnostic Device.
From www.regdesk.co
FDA Exemption for Class II Medical Devices RegDesk Fda Definition Of Diagnostic Device Manufacturers can find detailed information about complying with the. (2) the conditions of use for the device, including. (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. (1) the persons for whose use the device is represented or intended; An overview of how the fda regulates in vitro diagnostic products (ivd). You should read. Fda Definition Of Diagnostic Device.
From v9306.1blu.de
From PointofCare Testing To EHealth Diagnostic Devices Fda Definition Of Diagnostic Device Manufacturers can find detailed information about complying with the. An overview of how the fda regulates in vitro diagnostic products (ivd). (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: (2) the conditions of use for the device, including. The european medical devices regulation, uses a similar definition of a. Fda Definition Of Diagnostic Device.
From www.vrogue.co
Eu Ivd Approval Process For Medical Devices vrogue.co Fda Definition Of Diagnostic Device Manufacturers can find detailed information about complying with the. (a) in vitro diagnostic products are those reagents, instruments, and systems intended for use in. How does the eu mdr define a medical device? An overview of how the fda regulates in vitro diagnostic products (ivd). (1) the persons for whose use the device is represented or intended; (2) the conditions. Fda Definition Of Diagnostic Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Definition Of Diagnostic Device You should read this first. (1) the persons for whose use the device is represented or intended; The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use. Fda Definition Of Diagnostic Device.
From gioixjpns.blob.core.windows.net
Device Definition Fda at Brian Hudgens blog Fda Definition Of Diagnostic Device How does the eu mdr define a medical device? You should read this first. (2) the conditions of use for the device, including. An overview of how the fda regulates in vitro diagnostic products (ivd). Manufacturers can find detailed information about complying with the. (1) the persons for whose use the device is represented or intended; The european medical devices. Fda Definition Of Diagnostic Device.
From giodpatut.blob.core.windows.net
Medical Device System Definition at Danny Henke blog Fda Definition Of Diagnostic Device The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a. You should read this first. (1) the persons for whose use the device is represented. Fda Definition Of Diagnostic Device.
From www.greenlight.guru
Medical Device Classifications Determine Your Device Class Fda Definition Of Diagnostic Device (1) the persons for whose use the device is represented or intended; (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: You should read this first. How does the eu mdr define a medical device? The european medical devices regulation, uses a similar definition of a medical device to the. Fda Definition Of Diagnostic Device.
From www.scilife.io
In Vitro Diagnostics (IVD) A Complete Overview Scilife Fda Definition Of Diagnostic Device You should read this first. (1) the persons for whose use the device is represented or intended; (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. (a) in vitro diagnostic products. Fda Definition Of Diagnostic Device.
From medicaldeviceacademy.com
Overview of Similarities and Differences between QSR and ISO 13485 Fda Definition Of Diagnostic Device You should read this first. An overview of how the fda regulates in vitro diagnostic products (ivd). How does the eu mdr define a medical device? (1) the persons for whose use the device is represented or intended; (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: (a) in vitro. Fda Definition Of Diagnostic Device.
From www.thermofisher.cn
IVDD vs. IVDR Classifications Defined and Compared OEMpowered Fda Definition Of Diagnostic Device The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. How does the eu mdr define a medical device? (2) the conditions of use for the device, including. (1) the persons for whose use the device is represented or intended; An overview of how the fda regulates in vitro diagnostic products. Fda Definition Of Diagnostic Device.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Fda Definition Of Diagnostic Device How does the eu mdr define a medical device? You should read this first. Manufacturers can find detailed information about complying with the. (1) the persons for whose use the device is represented or intended; An overview of how the fda regulates in vitro diagnostic products (ivd). A companion diagnostic is a medical device, often an in vitro device, which. Fda Definition Of Diagnostic Device.
From www.medicaldevice-network.com
IVD market overview Medical Device Network Fda Definition Of Diagnostic Device The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: (2) the conditions of use for the device, including. (1) the persons for whose use the device is represented or intended; How. Fda Definition Of Diagnostic Device.
From gioixjpns.blob.core.windows.net
Device Definition Fda at Brian Hudgens blog Fda Definition Of Diagnostic Device (k) noninvasive, when applied to a diagnostic device or procedure, means one that does not by design or intention: The european medical devices regulation, uses a similar definition of a medical device to the fda and goes on. A companion diagnostic is a medical device, often an in vitro device, which provides information that is essential for the safe and. Fda Definition Of Diagnostic Device.