Formal Charge Chlorine Clf5 . Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. It exists as a colorless gas with a sweet odor. Seven minus 2 minus 5; Use the following formula to calculate the formal charges on atoms: For fluorine, they're all symmetrical, so we only need to do one. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Zero is the formal charge for chlorine. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). Here, both chlorine and fluorine atoms do not have charges. For that, you need to remember the formula of formal charge;
from carakruwlambert.blogspot.com
For fluorine, they're all symmetrical, so we only need to do one. Use the following formula to calculate the formal charges on atoms: Seven minus 2 minus 5; It exists as a colorless gas with a sweet odor. Zero is the formal charge for chlorine. For that, you need to remember the formula of formal charge; Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Here, both chlorine and fluorine atoms do not have charges. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges.
Calculate the Formal Charge on the Chlorine Cl Atom CarakruwLambert
Formal Charge Chlorine Clf5 Seven minus 2 minus 5; Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). For fluorine, they're all symmetrical, so we only need to do one. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. It exists as a colorless gas with a sweet odor. Seven minus 2 minus 5; Here, both chlorine and fluorine atoms do not have charges. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Zero is the formal charge for chlorine. Use the following formula to calculate the formal charges on atoms: For that, you need to remember the formula of formal charge;
From www.youtube.com
ClF3 Lewis Structure (Chlorine Trifluoride) YouTube Formal Charge Chlorine Clf5 Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Zero is the formal charge for chlorine. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Seven minus 2 minus 5; For that, you need to remember the formula of formal charge; For fluorine, they're all. Formal Charge Chlorine Clf5.
From www.coursehero.com
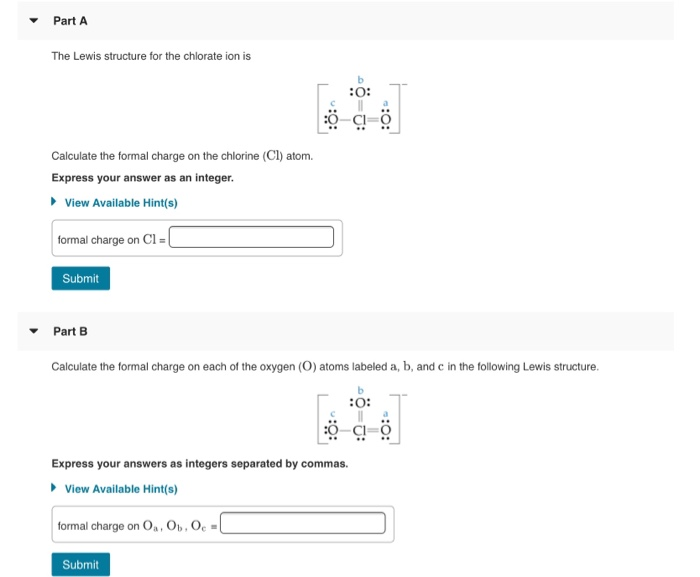
[Solved] Calculate the formal charge on the chlorine in chlorate Formal Charge Chlorine Clf5 Here, both chlorine and fluorine atoms do not have charges. Zero is the formal charge for chlorine. Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each. Formal Charge Chlorine Clf5.
From bilag.xxl.no
Draw The Lewis Structure For The Chlorine Pentafluoride Molecule Formal Charge Chlorine Clf5 It exists as a colorless gas with a sweet odor. Seven minus 2 minus 5; For that, you need to remember the formula of formal charge; For fluorine, they're all symmetrical, so we only need to do one. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Zero is the formal charge. Formal Charge Chlorine Clf5.
From techiescientist.com
ClF5 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Formal Charge Chlorine Clf5 Use the following formula to calculate the formal charges on atoms: Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. It exists as a colorless. Formal Charge Chlorine Clf5.
From www.youtube.com
ClF5 (Chlorine pentafluoride) Molecular Geometry, Bond Angles YouTube Formal Charge Chlorine Clf5 Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Zero is the formal charge for chlorine. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Seven minus 2 minus 5; It exists as a colorless gas with a sweet odor. Here, both chlorine and. Formal Charge Chlorine Clf5.
From www.chegg.com
Solved 3. What is the formal charge on the chlorine atom in Formal Charge Chlorine Clf5 For that, you need to remember the formula of formal charge; Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. For fluorine, they're all symmetrical, so we only need to do one. Zero is the formal charge for chlorine. Here, both chlorine and fluorine atoms do not have charges. Now, you. Formal Charge Chlorine Clf5.
From chem.libretexts.org
10.3 Compounds of Chlorine Chemistry LibreTexts Formal Charge Chlorine Clf5 It exists as a colorless gas with a sweet odor. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Use the following formula to calculate the formal charges on atoms: Zero is the formal charge for. Formal Charge Chlorine Clf5.
From www.chegg.com
Solved Calculate the formal charge for Chlorine. Then Formal Charge Chlorine Clf5 Seven minus 2 minus 5; Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Use the following formula to calculate the formal charges on atoms: Here, both chlorine and fluorine atoms do not have charges. For that, you need to remember the formula of formal charge; For fluorine, they're all symmetrical, so we. Formal Charge Chlorine Clf5.
From zeeshanodessa.blogspot.com
52+ calculate the formal charge on the chlorine cl atom ZeeshanOdessa Formal Charge Chlorine Clf5 Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Here, both chlorine and fluorine atoms do not have. Formal Charge Chlorine Clf5.
From www.youtube.com
ClF5 Lewis Structure How to Draw the Lewis Structure for ClF5 Formal Charge Chlorine Clf5 Use the following formula to calculate the formal charges on atoms: Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). Zero is the formal charge for chlorine. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of. Formal Charge Chlorine Clf5.
From www.chegg.com
Solved SA) What is the formal charge on the chlorine atom in Formal Charge Chlorine Clf5 Zero is the formal charge for chlorine. It exists as a colorless gas with a sweet odor. Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. For that, you need to remember the formula of formal charge; Now, you have come to the final step and here you have to check. Formal Charge Chlorine Clf5.
From wisc.pb.unizin.org
M8Q3 Resonance Structures and Formal Charge Chem 103/104 Resource Book Formal Charge Chlorine Clf5 Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). For fluorine, they're all symmetrical, so we only need to do one. Use the following formula. Formal Charge Chlorine Clf5.
From www.youtube.com
Hybridization of ClF5 (Chlorine pentafluoride) YouTube Formal Charge Chlorine Clf5 For that, you need to remember the formula of formal charge; Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Use the following formula to calculate the formal charges on atoms: Now, you have come to the final step and here you have to check the formal charge on chlorine atom. Formal Charge Chlorine Clf5.
From www.youtube.com
How to Draw the Lewis Structure for ClO2 (Chlorine dioxide) YouTube Formal Charge Chlorine Clf5 For fluorine, they're all symmetrical, so we only need to do one. Zero is the formal charge for chlorine. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each. Formal Charge Chlorine Clf5.
From www.chegg.com
Solved Part A Calculate the formal charge on the chlorine Formal Charge Chlorine Clf5 It exists as a colorless gas with a sweet odor. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Use the following formula to calculate the formal charges on atoms: Here, both chlorine and fluorine atoms do not have charges. Zero is the formal charge for chlorine. For that, you need to. Formal Charge Chlorine Clf5.
From h-o-m-e.org
The Formal Charge in Chlorine's Most Common Form Perchlorate Formal Charge Chlorine Clf5 Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). For that, you need to remember the formula of formal charge; Let’s verify the stability. Formal Charge Chlorine Clf5.
From carakruwlambert.blogspot.com
Calculate the Formal Charge on the Chlorine Cl Atom CarakruwLambert Formal Charge Chlorine Clf5 Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. For that, you need to remember the formula of formal charge; It exists as a colorless gas with a sweet odor. Here, both chlorine and. Formal Charge Chlorine Clf5.
From www.chegg.com
Solved The formal charge on Cl in the structure shown for Formal Charge Chlorine Clf5 Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of. Formal Charge Chlorine Clf5.
From www.numerade.com
SOLVED 'Cl Q' For the Lewis diagram, above, determine The formal Formal Charge Chlorine Clf5 Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Here, both chlorine and fluorine atoms do not have charges. For fluorine, they're all symmetrical, so we only need to do one. Zero is the formal charge for chlorine. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal. Formal Charge Chlorine Clf5.
From zeeshanodessa.blogspot.com
52+ calculate the formal charge on the chlorine cl atom ZeeshanOdessa Formal Charge Chlorine Clf5 Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Seven minus 2 minus 5; It exists as a colorless gas with a sweet odor. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f).. Formal Charge Chlorine Clf5.
From www.chegg.com
Solved What is the formal charge of the Chlorine and Formal Charge Chlorine Clf5 Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). Use the following formula to calculate the formal charges on atoms: Here, both chlorine and fluorine atoms do not have charges. For fluorine, they're all symmetrical, so we only need to do. Formal Charge Chlorine Clf5.
From www.youtube.com
ClF3 Lewis Structure How to Draw the Lewis Structure for ClF3 YouTube Formal Charge Chlorine Clf5 Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). It exists as a colorless gas with a sweet odor. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Use the following formula to. Formal Charge Chlorine Clf5.
From www.chegg.com
Solved What is the formal charge of the chlorine atom in Formal Charge Chlorine Clf5 Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). For that, you need to remember the formula of formal charge; Zero is the formal. Formal Charge Chlorine Clf5.
From www.numerade.com
SOLVED a. Determine the formal charge on the chlorine atom in the Formal Charge Chlorine Clf5 Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Seven minus 2 minus 5; Use the following formula to calculate the formal charges on atoms: For that, you need to remember the formula of formal. Formal Charge Chlorine Clf5.
From www.alamy.com
Chlorine pentafluoride is an interhalogen compound with formula ClF5 Formal Charge Chlorine Clf5 For fluorine, they're all symmetrical, so we only need to do one. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Use the following formula to calculate the formal charges on atoms: Here, both chlorine and fluorine atoms do not have charges. For that, you need to remember the formula of formal charge;. Formal Charge Chlorine Clf5.
From h-o-m-e.org
The Formal Charge in Chlorine's Most Common Form Perchlorate Formal Charge Chlorine Clf5 It exists as a colorless gas with a sweet odor. Seven minus 2 minus 5; Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Zero is the formal charge for chlorine. Chlorine pentafluoride (clf5) lewis. Formal Charge Chlorine Clf5.
From www.chegg.com
Solved 1. (1 pt) Calculate the formal charges on chlorine in Formal Charge Chlorine Clf5 Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Here, both chlorine and fluorine atoms do not have charges. For that, you need to remember the formula of formal charge; Zero is the formal charge for chlorine. Seven minus 2 minus 5; Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron. Formal Charge Chlorine Clf5.
From www.youtube.com
7.53 Calculate the formal charge of chlorine in the molecules Cl2 Formal Charge Chlorine Clf5 Seven minus 2 minus 5; Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Zero is the formal charge for chlorine. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f). It exists as a. Formal Charge Chlorine Clf5.
From www.chemtube3d.com
ClF5 Chlorine pentafluoride Formal Charge Chlorine Clf5 It exists as a colorless gas with a sweet odor. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. For that, you need to remember the formula of formal charge; Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as. Formal Charge Chlorine Clf5.
From byjus.com
What is the Formal charge on Cl in HClO4? Formal Charge Chlorine Clf5 For that, you need to remember the formula of formal charge; Here, both chlorine and fluorine atoms do not have charges. Use the following formula to calculate the formal charges on atoms: Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine atom (f).. Formal Charge Chlorine Clf5.
From www.numerade.com
SOLVED Consider the first Lewis structure for NOCl The formal charge Formal Charge Chlorine Clf5 Seven minus 2 minus 5; It exists as a colorless gas with a sweet odor. For that, you need to remember the formula of formal charge; Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge,. Formal Charge Chlorine Clf5.
From ar.inspiredpencil.com
Clf5 Lewis Dot Structure Formal Charge Chlorine Clf5 Zero is the formal charge for chlorine. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. It exists as a colorless gas with a sweet odor. Use the following formula to calculate the formal. Formal Charge Chlorine Clf5.
From ezra-kwells.blogspot.com
Calculate the Formal Charge on the Chlorine Cl Atom Formal Charge Chlorine Clf5 Use the following formula to calculate the formal charges on atoms: For that, you need to remember the formula of formal charge; For fluorine, they're all symmetrical, so we only need to do one. Now, you have come to the final step and here you have to check the formal charge on chlorine atom (cl) as well as each fluorine. Formal Charge Chlorine Clf5.
From www.youtube.com
What is the formal charge of chlorine in the molecules Cl2, BeCl2, and Formal Charge Chlorine Clf5 Use the following formula to calculate the formal charges on atoms: For fluorine, they're all symmetrical, so we only need to do one. Let’s verify the stability of the chlorine pentafluoride lewis structure using the concept of formal charges. Chlorine pentafluoride (clf5) lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. Here, both chlorine and. Formal Charge Chlorine Clf5.
From byjus.com
Calculate the formal charge on Cl atom in HCIO4. Formal Charge Chlorine Clf5 Seven minus 2 minus 5; For fluorine, they're all symmetrical, so we only need to do one. Clf 5 is the chemical formula for an interhalogen compound known as penta fluoro chlorine or chlorine pentafluoride. It exists as a colorless gas with a sweet odor. Now, you have come to the final step and here you have to check the. Formal Charge Chlorine Clf5.