Initial Burette Reading And Final . In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. A typical layout and set of titration results. Take an initial reading of the burette. Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; Using a thermometer for the measurement of the temperature at the start. Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible and the titration technique is. Using the methods above, take and record an initial reading of the liquid in your burette to. The difference between the first and final reading is called the titer. The first and final reading of the burette should be recorded. Using a balance to measure the initial and final mass of a container; Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration.
from www.numerade.com
Using a balance to measure the initial and final mass of a container; In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible and the titration technique is. The difference between the first and final reading is called the titer. Take an initial reading of the burette. Using a thermometer for the measurement of the temperature at the start. The first and final reading of the burette should be recorded. A typical layout and set of titration results. Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty;
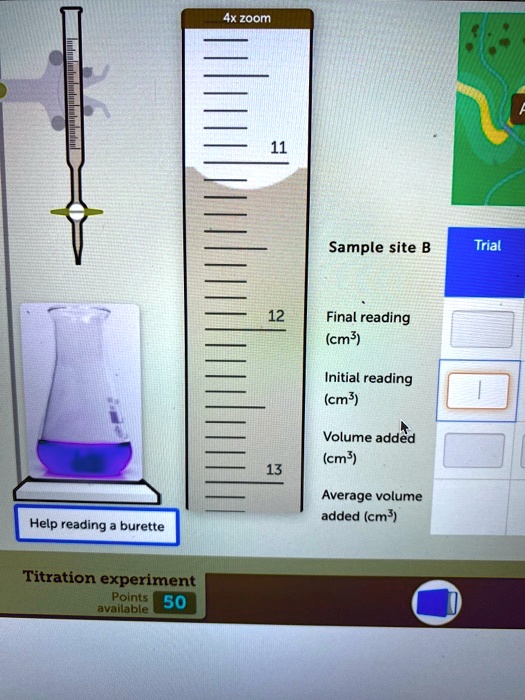
SOLVEDAx zoom Sample site B Trial Final reading (cm ) Initial reading
Initial Burette Reading And Final Take an initial reading of the burette. Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; The first and final reading of the burette should be recorded. Take an initial reading of the burette. The difference between the first and final reading is called the titer. Using a thermometer for the measurement of the temperature at the start. A typical layout and set of titration results. In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. Using the methods above, take and record an initial reading of the liquid in your burette to. Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. Using a balance to measure the initial and final mass of a container; Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible and the titration technique is. Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration.
From www.numerade.com
SOLVED Three titrations were done. Parts of the burette with liquid Initial Burette Reading And Final Using a thermometer for the measurement of the temperature at the start. Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. A typical layout and set of titration results. Take an initial reading of the burette. The difference between the first and final reading is called the. Initial Burette Reading And Final.
From www.numerade.com
SOLVEDAx zoom Sample site B Trial Final reading (cm ) Initial reading Initial Burette Reading And Final Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. Using a balance to measure the initial and final mass of a container; Using a thermometer for the measurement of the temperature at the start. Take an initial reading of the burette. Using the methods above, take and. Initial Burette Reading And Final.
From www.numerade.com
SOLVED The diagram of a burette shown below shows the initial and Initial Burette Reading And Final Take an initial reading of the burette. Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. Using the methods above, take and record. Initial Burette Reading And Final.
From learningmagicbates88.z13.web.core.windows.net
Reading A Burette Worksheet Initial Burette Reading And Final Take an initial reading of the burette. Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. A typical layout and set of titration results. Using a balance to measure the initial and final mass of a container; Both the initial and final burette readings should be. Initial Burette Reading And Final.
From www.chegg.com
Solved Experimental Results Initial Burette Reading (mL) Initial Burette Reading And Final Using the methods above, take and record an initial reading of the liquid in your burette to. In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. Using a thermometer for the measurement of the temperature at the start. Lf 2 or 3 values are. Initial Burette Reading And Final.
From www.coursehero.com
[Solved] Acid &base Titration Sample video link... Course Hero Initial Burette Reading And Final In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. The difference between the first and final reading is called the titer. Using. Initial Burette Reading And Final.
From www.numerade.com
SOLVEDTrial 1 (FAST) 2 (SLOW) Final burette reading (mL) 15lil 0 Initial Burette Reading And Final Take an initial reading of the burette. Using the methods above, take and record an initial reading of the liquid in your burette to. Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; In this experiment, you will learn how to read and manipulate a. Initial Burette Reading And Final.
From worksheetlistwu.z13.web.core.windows.net
Burette Reading Worksheet Initial Burette Reading And Final Using a balance to measure the initial and final mass of a container; A typical layout and set of titration results. Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. Take an initial reading of the burette. Using a thermometer for the measurement of the temperature at. Initial Burette Reading And Final.
From www.numerade.com
SOLVED By performing an iteration, we are able to determine the titer Initial Burette Reading And Final Using a thermometer for the measurement of the temperature at the start. A typical layout and set of titration results. Take an initial reading of the burette. In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. Lf 2 or 3 values are within 0.10cm3. Initial Burette Reading And Final.
From www.numerade.com
SOLVED Observations Standardization of NaOH SI. No Volume of Oxalic Initial Burette Reading And Final The first and final reading of the burette should be recorded. Take an initial reading of the burette. A typical layout and set of titration results. Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; Using a thermometer for the measurement of the temperature at. Initial Burette Reading And Final.
From www.chegg.com
Solved bo Initial Burette reading of Na2S2O3 Final Burette Initial Burette Reading And Final The difference between the first and final reading is called the titer. Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible and the titration technique is. Using a. Initial Burette Reading And Final.
From www.chegg.com
Solved initial burette reading/cma final burette reading/cm3 Initial Burette Reading And Final A typical layout and set of titration results. Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. Read the bottom of the. Initial Burette Reading And Final.
From www.chegg.com
Solved 1.0 Initial burette reading (mL) Final burette Initial Burette Reading And Final A typical layout and set of titration results. Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. The difference between the first and final reading is called the titer. The first and final reading of the burette should be recorded. In this experiment, you will learn. Initial Burette Reading And Final.
From www.coursehero.com
[Solved] Question 17 of 17 Consider the following image of two buret Initial Burette Reading And Final Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; A typical layout and set of titration results. In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. The difference between. Initial Burette Reading And Final.
From www.chegg.com
Solved Trial 1 Initial Read the following burette Initial Burette Reading And Final Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. Take an initial reading of the burette. The difference between the first and final reading. Initial Burette Reading And Final.
From www.doubtnut.com
The initial and final reading of a burette while draining out 50 drops Initial Burette Reading And Final Using a thermometer for the measurement of the temperature at the start. Using the methods above, take and record an initial reading of the liquid in your burette to. In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. Read the bottom of the meniscus. Initial Burette Reading And Final.
From www.numerade.com
SOLVED Introduction to Titrations Data Sheet Data TableTitntion Pan k Initial Burette Reading And Final Using the methods above, take and record an initial reading of the liquid in your burette to. The first and final reading of the burette should be recorded. Using a balance to measure the initial and final mass of a container; Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible. Initial Burette Reading And Final.
From www.animalia-life.club
Reading A Buret Initial Burette Reading And Final Take an initial reading of the burette. Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. The first and final reading of the burette should be recorded. Using a thermometer for the measurement of the temperature at the start. A typical layout and set of titration. Initial Burette Reading And Final.
From learningschoolbrady88.z13.web.core.windows.net
Reading A Burrette Practice Worksheet Initial Burette Reading And Final In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. The difference between the first and final reading is called the titer.. Initial Burette Reading And Final.
From www.chegg.com
Solved TRIAL A Initial Burette Reading (mL) 0.500 mL Final Initial Burette Reading And Final Take an initial reading of the burette. Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. Using the methods above, take and record. Initial Burette Reading And Final.
From www.coursehero.com
[Solved] Question 17 of 17 Consider the following image of two buret Initial Burette Reading And Final Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. Using a balance to measure the initial and final mass of a container; Using the methods above, take and record an initial reading of the liquid in your burette to. In this experiment, you will learn how to. Initial Burette Reading And Final.
From www.chegg.com
Solved Using NaCl Final burette reading 25.90mL Initial Initial Burette Reading And Final The first and final reading of the burette should be recorded. Using a balance to measure the initial and final mass of a container; Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. Take an initial reading of the burette. Both the initial and final burette. Initial Burette Reading And Final.
From www.chegg.com
Trial 1 Volume of HCl sample Final buret reading, Initial Burette Reading And Final Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; A typical layout and set of titration results. Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible and the titration technique is. Using the methods above,. Initial Burette Reading And Final.
From slidetodoc.com
10 2 Neutralization and AcidBase Titrations Learning Goals Initial Burette Reading And Final Using the methods above, take and record an initial reading of the liquid in your burette to. The first and final reading of the burette should be recorded. Take an initial reading of the burette. Using a thermometer for the measurement of the temperature at the start. The difference between the first and final reading is called the titer. A. Initial Burette Reading And Final.
From www.chegg.com
Solved Read the buret (burette) volume and report your Initial Burette Reading And Final Take an initial reading of the burette. Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; Lf 2 or 3 values are. Initial Burette Reading And Final.
From www.chegg.com
Solved Experimental Results Initial Burette Reading (mL) Initial Burette Reading And Final The difference between the first and final reading is called the titer. Using a balance to measure the initial and final mass of a container; Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. Record the initial burette reading (to 2 decimal places) before titration, and. Initial Burette Reading And Final.
From www.chegg.com
Solved Lab 8 Day2 Part B Initial burette reading (ML) Final Initial Burette Reading And Final Using a thermometer for the measurement of the temperature at the start. Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. The first and final reading of the burette should be recorded. Take an initial reading of the burette. Using a balance to measure the initial and. Initial Burette Reading And Final.
From www.quelpr.com
CSEC Chemistry Volumetric Analysis Initial Burette Reading And Final Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible and the titration technique is. In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. The first and final reading of the burette should be recorded.. Initial Burette Reading And Final.
From www.numerade.com
SOLVED Observations/Results Qualitive and Quantitative Table 1 Initial Burette Reading And Final Take an initial reading of the burette. Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end of the titration. Using a thermometer for the measurement of the temperature at the start. Using a balance to measure the initial and final mass of a container; In this experiment, you will learn. Initial Burette Reading And Final.
From chem4three.blogspot.com
CHEMISTRY 11 February 2011 Initial Burette Reading And Final Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible and the titration technique is. Using a balance to measure the initial and final mass of a. Initial Burette Reading And Final.
From drawingrick.blogspot.com
Reading A Burrette Practice Worksheet / Lesson Worksheet Measurement Initial Burette Reading And Final A typical layout and set of titration results. The difference between the first and final reading is called the titer. Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible and the titration technique is. In this experiment, you will learn how to read and manipulate a burette in a technique. Initial Burette Reading And Final.
From www.chegg.com
Solved SECOND TITRATION Sodium Hydroxide Final burette Initial Burette Reading And Final Using a thermometer for the measurement of the temperature at the start. Take an initial reading of the burette. In this experiment, you will learn how to read and manipulate a burette in a technique called titration and determine the unknown concentration of a. The first and final reading of the burette should be recorded. Using a balance to measure. Initial Burette Reading And Final.
From mmerevise.co.uk
Acids and Bases Questions and Revision MME Initial Burette Reading And Final Using the methods above, take and record an initial reading of the liquid in your burette to. Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible and the titration technique is. Record the initial burette reading (to 2 decimal places) before titration, and the final burette reading at the end. Initial Burette Reading And Final.
From www.numerade.com
SOLVED Part B Acid solution pipetted 5.00 mL; Burette readings (NaOH Initial Burette Reading And Final The difference between the first and final reading is called the titer. Lf 2 or 3 values are within 0.10cm3 and are therefore concordant, then the results are accurate and reproducible and the titration technique is. Using the methods above, take and record an initial reading of the liquid in your burette to. Both the initial and final burette readings. Initial Burette Reading And Final.
From www.numerade.com
SOLVED The student repeated the titration experiment three times. The Initial Burette Reading And Final Read the bottom of the meniscus on the burette this is reading 9.00cm3 even though a burette has marking reading to 0.1cm3, the. A typical layout and set of titration results. Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm 3, the same as the uncertainty; Take an initial reading of. Initial Burette Reading And Final.