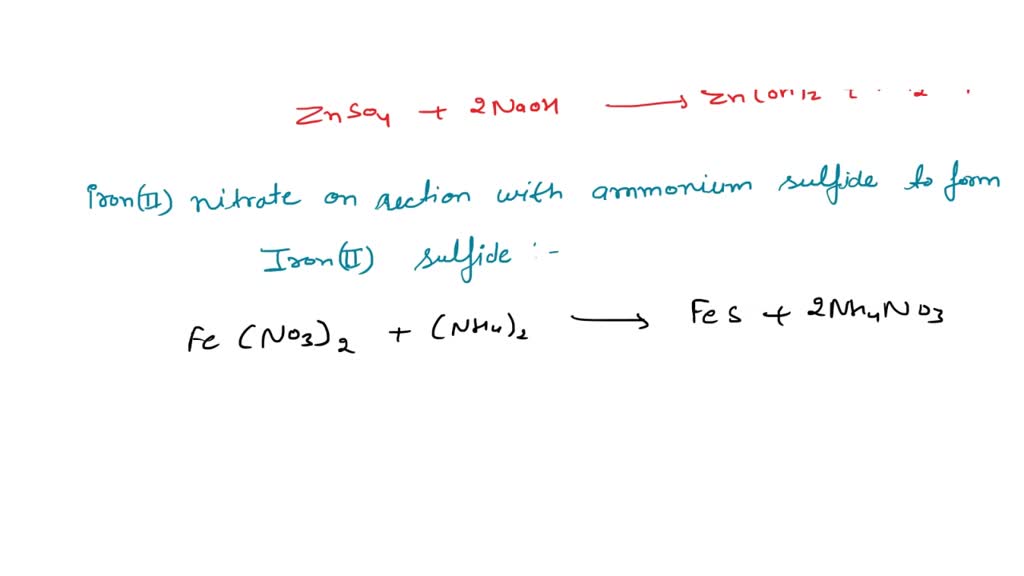
Potassium Hydroxide And Zinc Sulfate Precipitate . Recognize and identify examples of precipitation reactions. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. We described a precipitation reaction in which a colorless solution of. Znso4 + koh = zn(oh)2 + k2so4 is a double displacement (metathesis) reaction where one mole of aqueous zinc sulfate [znso 4] and two. This guide will show how to use the solubility rules. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. Apply the solubility rules of common inorganic compounds to predict the products. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. How many grams of zinc(ii) nitrate and sodium sulfide. You can use ammonia solution instead of sodium hydroxide. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution.
from www.numerade.com
7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. You can use ammonia solution instead of sodium hydroxide. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Enter an equation of an ionic chemical equation and press the balance button. Znso4 + koh = zn(oh)2 + k2so4 is a double displacement (metathesis) reaction where one mole of aqueous zinc sulfate [znso 4] and two. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. This guide will show how to use the solubility rules. We described a precipitation reaction in which a colorless solution of. The balanced equation will be calculated along with the.
SOLVED Does precipitate form when A and B are mixed? empirical formula
Potassium Hydroxide And Zinc Sulfate Precipitate This guide will show how to use the solubility rules. Apply the solubility rules of common inorganic compounds to predict the products. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. This guide will show how to use the solubility rules. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Znso4 + koh = zn(oh)2 + k2so4 is a double displacement (metathesis) reaction where one mole of aqueous zinc sulfate [znso 4] and two. Recognize and identify examples of precipitation reactions. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. The balanced equation will be calculated along with the. How many grams of zinc(ii) nitrate and sodium sulfide. Enter an equation of an ionic chemical equation and press the balance button. We described a precipitation reaction in which a colorless solution of. You can use ammonia solution instead of sodium hydroxide. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Potassium Hydroxide And Zinc Sulfate Precipitate A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. How many grams of zinc(ii) nitrate and sodium sulfide. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. Recognize and identify examples of precipitation reactions. We described a precipitation. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Potassium Hydroxide And Zinc Sulfate Precipitate Enter an equation of an ionic chemical equation and press the balance button. How many grams of zinc(ii) nitrate and sodium sulfide. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. The balanced equation will be calculated along with the. Recognize and identify examples of. Potassium Hydroxide And Zinc Sulfate Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Potassium Hydroxide And Zinc Sulfate Precipitate Enter an equation of an ionic chemical equation and press the balance button. Znso4 + koh = zn(oh)2 + k2so4 is a double displacement (metathesis) reaction where one mole of aqueous zinc sulfate [znso 4] and two. The balanced equation will be calculated along with the. You can use ammonia solution instead of sodium hydroxide. We described a precipitation reaction. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVEDFor following double displacement reactions use the Solubility Potassium Hydroxide And Zinc Sulfate Precipitate Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Apply the solubility rules of common inorganic compounds to predict the products. We described a precipitation reaction in which a colorless solution of. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.youtube.com
Reaction of Iron (II) Sulphate (FeSO4) with Sodium Hydroxide (NaOH Potassium Hydroxide And Zinc Sulfate Precipitate A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Apply the solubility rules of common inorganic compounds to predict the products. 7 rows solutions containing copper (ii). Potassium Hydroxide And Zinc Sulfate Precipitate.
From ocdrum.com
nickel(ii iodide and potassium carbonate balanced equation) Potassium Hydroxide And Zinc Sulfate Precipitate This guide will show how to use the solubility rules. You can use ammonia solution instead of sodium hydroxide. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Enter an equation of an ionic chemical equation and press the balance button. Recognize and identify examples of precipitation reactions. Znso4 + koh =. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.youtube.com
Reaction between copper(II)sulfate and potassium hydroxide YouTube Potassium Hydroxide And Zinc Sulfate Precipitate We described a precipitation reaction in which a colorless solution of. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Recognize and identify examples of precipitation reactions. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. A vivid. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED For each proposed mechanism, choose a reagent (zinc sulfate Potassium Hydroxide And Zinc Sulfate Precipitate Recognize and identify examples of precipitation reactions. This guide will show how to use the solubility rules. Enter an equation of an ionic chemical equation and press the balance button. Apply the solubility rules of common inorganic compounds to predict the products. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.youtube.com
Does zinc sulfate (ZnSO4) and potassium sulfide (K2S) form a Potassium Hydroxide And Zinc Sulfate Precipitate This guide will show how to use the solubility rules. Znso4 + koh = zn(oh)2 + k2so4 is a double displacement (metathesis) reaction where one mole of aqueous zinc sulfate [znso 4] and two. The balanced equation will be calculated along with the. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution.. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.sciencephoto.com
Metal Hydroxide Precipitates Stock Image C027/9452 Science Photo Potassium Hydroxide And Zinc Sulfate Precipitate Enter an equation of an ionic chemical equation and press the balance button. This guide will show how to use the solubility rules. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Does the empirical formula of a precipitate form when A and B Potassium Hydroxide And Zinc Sulfate Precipitate Enter an equation of an ionic chemical equation and press the balance button. This guide will show how to use the solubility rules. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.toppr.com
Translate the following statements into chemical equations and then Potassium Hydroxide And Zinc Sulfate Precipitate Apply the solubility rules of common inorganic compounds to predict the products. You can use ammonia solution instead of sodium hydroxide. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Complete the table below by deciding whether precipitate forms Potassium Hydroxide And Zinc Sulfate Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. This guide will show how to use the solubility rules. We described a precipitation reaction in which a colorless solution of. Znso4 + koh = zn(oh)2 + k2so4 is a double displacement (metathesis) reaction where one mole of aqueous zinc sulfate [znso. Potassium Hydroxide And Zinc Sulfate Precipitate.
From ar.inspiredpencil.com
Zinc Hydroxide Potassium Hydroxide And Zinc Sulfate Precipitate You can use ammonia solution instead of sodium hydroxide. This guide will show how to use the solubility rules. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. The balanced equation will be calculated along with the. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.youtube.com
Does Potassium hydroxide (KOH) and Zinc sulfate (ZnSO4) form a Potassium Hydroxide And Zinc Sulfate Precipitate Apply the solubility rules of common inorganic compounds to predict the products. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. We described a precipitation reaction in which a colorless solution of. This guide will show how to use the solubility rules. Znso4 + koh. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Texts Complete the table below by deciding whether a Potassium Hydroxide And Zinc Sulfate Precipitate This guide will show how to use the solubility rules. The balanced equation will be calculated along with the. We described a precipitation reaction in which a colorless solution of. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. A vivid example of precipitation is observed when solutions of potassium iodide. Potassium Hydroxide And Zinc Sulfate Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Potassium Hydroxide And Zinc Sulfate Precipitate Recognize and identify examples of precipitation reactions. Apply the solubility rules of common inorganic compounds to predict the products. You can use ammonia solution instead of sodium hydroxide. Enter an equation of an ionic chemical equation and press the balance button. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. A. Potassium Hydroxide And Zinc Sulfate Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Potassium Hydroxide And Zinc Sulfate Precipitate We described a precipitation reaction in which a colorless solution of. Enter an equation of an ionic chemical equation and press the balance button. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Recognize. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Complete the table below by deciding whether precipitate forms Potassium Hydroxide And Zinc Sulfate Precipitate You can use ammonia solution instead of sodium hydroxide. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. How many grams of zinc(ii) nitrate and sodium sulfide. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Adding 10.0. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVEDsolid zinc hydrochloric acid hydrogen gas aqueous zinc chloride Potassium Hydroxide And Zinc Sulfate Precipitate A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. You can use ammonia solution instead of sodium hydroxide. Znso4 + koh = zn(oh)2 + k2so4 is a double displacement (metathesis) reaction where one mole of aqueous zinc sulfate [znso 4] and two. Enter an equation. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.youtube.com
BaCl2+K2SO4 Barium chloride + Potassium sulfate YouTube Potassium Hydroxide And Zinc Sulfate Precipitate We described a precipitation reaction in which a colorless solution of. Enter an equation of an ionic chemical equation and press the balance button. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.youtube.com
NaOH + MgSO4 Reaction & Precipitate Blossoms 💮 YouTube Potassium Hydroxide And Zinc Sulfate Precipitate Apply the solubility rules of common inorganic compounds to predict the products. The balanced equation will be calculated along with the. This guide will show how to use the solubility rules. How many grams of zinc(ii) nitrate and sodium sulfide. Znso4 + koh = zn(oh)2 + k2so4 is a double displacement (metathesis) reaction where one mole of aqueous zinc sulfate. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Complete the table below bv deciding whether precipitate forms Potassium Hydroxide And Zinc Sulfate Precipitate Enter an equation of an ionic chemical equation and press the balance button. This guide will show how to use the solubility rules. How many grams of zinc(ii) nitrate and sodium sulfide. The balanced equation will be calculated along with the. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. A vivid example. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.chegg.com
Solved solution A solution B Does a precipitate form when A Potassium Hydroxide And Zinc Sulfate Precipitate You can use ammonia solution instead of sodium hydroxide. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. This guide will show how to use the solubility rules. Znso4 + koh = zn(oh)2 + k2so4 is a double displacement (metathesis) reaction where one mole of aqueous zinc sulfate [znso 4] and two.. Potassium Hydroxide And Zinc Sulfate Precipitate.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Potassium Hydroxide And Zinc Sulfate Precipitate Enter an equation of an ionic chemical equation and press the balance button. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The balanced equation will be calculated along with the. How many grams of zinc(ii) nitrate and sodium sulfide. This guide will show how to use the solubility rules. A vivid. Potassium Hydroxide And Zinc Sulfate Precipitate.
From ramonnewscook.blogspot.com
Zinc Nitrate + Sodium Carbonate Balanced Equation Potassium Hydroxide And Zinc Sulfate Precipitate We described a precipitation reaction in which a colorless solution of. This guide will show how to use the solubility rules. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Recognize and identify examples of precipitation reactions. The balanced equation will be calculated along with the. When two aqueous solutions of ionic. Potassium Hydroxide And Zinc Sulfate Precipitate.
From chemistry-chemists.com
Copper sulphate, potassium hydroxide and aluminium. Сульфат меди Potassium Hydroxide And Zinc Sulfate Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. This guide will show how to use the solubility rules. You can use ammonia solution instead of sodium hydroxide. Recognize and identify examples of precipitation reactions. The balanced equation will be calculated along with the. A precipitation reaction is a reaction that. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Does precipitate form when A and B are mixed? empirical formula Potassium Hydroxide And Zinc Sulfate Precipitate The balanced equation will be calculated along with the. Recognize and identify examples of precipitation reactions. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. You can use. Potassium Hydroxide And Zinc Sulfate Precipitate.
From ar.inspiredpencil.com
Zinc Hydroxide Potassium Hydroxide And Zinc Sulfate Precipitate Enter an equation of an ionic chemical equation and press the balance button. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. We described a precipitation reaction in which a colorless solution of. You can use ammonia solution instead of sodium hydroxide. How many grams. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED System 6 Double Replacement Reactions lead(I) nitrate and Potassium Hydroxide And Zinc Sulfate Precipitate The balanced equation will be calculated along with the. This guide will show how to use the solubility rules. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution.. Potassium Hydroxide And Zinc Sulfate Precipitate.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Potassium Hydroxide And Zinc Sulfate Precipitate Enter an equation of an ionic chemical equation and press the balance button. Recognize and identify examples of precipitation reactions. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. We described a precipitation reaction in which a colorless solution of. Znso4 + koh = zn(oh)2. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Potassium Hydroxide And Zinc Sulfate Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. Apply the solubility rules of common inorganic compounds to predict the products. This guide will show how to use the solubility rules. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. Enter an equation of an. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Construct A hand written solution for the following question Potassium Hydroxide And Zinc Sulfate Precipitate A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. You can use ammonia solution instead of sodium hydroxide. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Znso4 + koh = zn(oh)2 + k2so4 is a double. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.researchgate.net
a Color change and formation of white precipitate, b mechanism of Potassium Hydroxide And Zinc Sulfate Precipitate You can use ammonia solution instead of sodium hydroxide. The balanced equation will be calculated along with the. Znso4 + koh = zn(oh)2 + k2so4 is a double displacement (metathesis) reaction where one mole of aqueous zinc sulfate [znso 4] and two. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Adding. Potassium Hydroxide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED 'Does a empirical formula of precipitate precipitate form when Potassium Hydroxide And Zinc Sulfate Precipitate Enter an equation of an ionic chemical equation and press the balance button. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. The balanced equation will be calculated along with the. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of. Potassium Hydroxide And Zinc Sulfate Precipitate.