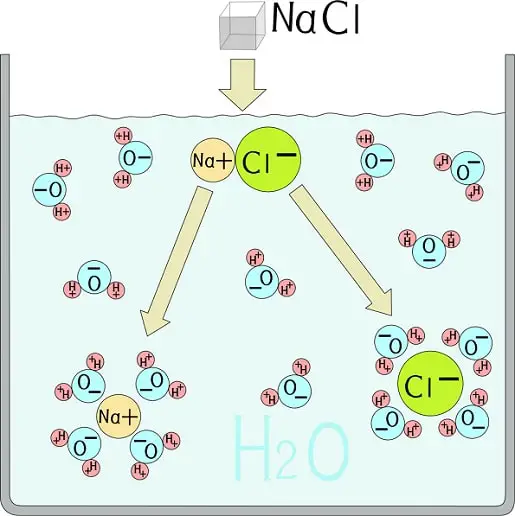
Chloride Ion Would Be An Example . Although chlorine as an element is a diatomic molecule, cl 2 , elemental. For example, it is present in the amylase enzyme. an ionic compound is an electrically neutral compound consisting of positive and negative ions. sodium chloride, or table salt, is an ionic compound. a chloride ion is a structural component of some proteins; To this end, chlorides are widely defined as any. Let’s take a look at how it is formed. You are very familiar with some ionic compounds. there are two chloride ions in the formula. During the formation of sodium. chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. This means that the one iron ion must have a.
from www.studyread.com
an ionic compound is an electrically neutral compound consisting of positive and negative ions. For example, it is present in the amylase enzyme. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. During the formation of sodium. sodium chloride, or table salt, is an ionic compound. Let’s take a look at how it is formed. To this end, chlorides are widely defined as any. You are very familiar with some ionic compounds. a chloride ion is a structural component of some proteins;
Difference between Ion and Atom in Points with Examples
Chloride Ion Would Be An Example For example, it is present in the amylase enzyme. a chloride ion is a structural component of some proteins; an ionic compound is an electrically neutral compound consisting of positive and negative ions. This means that the one iron ion must have a. During the formation of sodium. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. sodium chloride, or table salt, is an ionic compound. To this end, chlorides are widely defined as any. You are very familiar with some ionic compounds. there are two chloride ions in the formula. chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. Let’s take a look at how it is formed. For example, it is present in the amylase enzyme.
From mavink.com
Sodium Chloride Dot And Cross Diagram Chloride Ion Would Be An Example You are very familiar with some ionic compounds. sodium chloride, or table salt, is an ionic compound. For example, it is present in the amylase enzyme. Let’s take a look at how it is formed. During the formation of sodium. a chloride ion is a structural component of some proteins; there are two chloride ions in the. Chloride Ion Would Be An Example.
From mungfali.com
Sodium Chloride Molecule Diagram Chloride Ion Would Be An Example sodium chloride, or table salt, is an ionic compound. Let’s take a look at how it is formed. For example, it is present in the amylase enzyme. You are very familiar with some ionic compounds. a chloride ion is a structural component of some proteins; This means that the one iron ion must have a. Although chlorine as. Chloride Ion Would Be An Example.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive Chloride Ion Would Be An Example This means that the one iron ion must have a. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. there are two chloride ions in the formula. an ionic compound is an electrically neutral compound consisting of positive and negative ions. During the formation of sodium. You are very familiar with some ionic compounds.. Chloride Ion Would Be An Example.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Chloride Ion Would Be An Example Although chlorine as an element is a diatomic molecule, cl 2 , elemental. You are very familiar with some ionic compounds. To this end, chlorides are widely defined as any. Let’s take a look at how it is formed. there are two chloride ions in the formula. This means that the one iron ion must have a. sodium. Chloride Ion Would Be An Example.
From mammothmemory.net
Chemical bonding is about atoms achieving full outer shells Chloride Ion Would Be An Example Let’s take a look at how it is formed. sodium chloride, or table salt, is an ionic compound. an ionic compound is an electrically neutral compound consisting of positive and negative ions. To this end, chlorides are widely defined as any. there are two chloride ions in the formula. You are very familiar with some ionic compounds.. Chloride Ion Would Be An Example.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chloride Ion Would Be An Example During the formation of sodium. a chloride ion is a structural component of some proteins; sodium chloride, or table salt, is an ionic compound. chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. To this end, chlorides are widely defined as any. For example, it is present. Chloride Ion Would Be An Example.
From www.studyread.com
Difference between Ion and Atom in Points with Examples Chloride Ion Would Be An Example a chloride ion is a structural component of some proteins; To this end, chlorides are widely defined as any. an ionic compound is an electrically neutral compound consisting of positive and negative ions. During the formation of sodium. chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−.. Chloride Ion Would Be An Example.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Chloride Ion Would Be An Example For example, it is present in the amylase enzyme. an ionic compound is an electrically neutral compound consisting of positive and negative ions. This means that the one iron ion must have a. there are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. chloride always has. Chloride Ion Would Be An Example.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chloride Ion Would Be An Example an ionic compound is an electrically neutral compound consisting of positive and negative ions. For example, it is present in the amylase enzyme. Let’s take a look at how it is formed. To this end, chlorides are widely defined as any. You are very familiar with some ionic compounds. chloride always has a 1− charge, so with two. Chloride Ion Would Be An Example.
From schematicdatajudder88.z22.web.core.windows.net
Chlorine Atomic Structure Diagram Chloride Ion Would Be An Example This means that the one iron ion must have a. there are two chloride ions in the formula. an ionic compound is an electrically neutral compound consisting of positive and negative ions. Let’s take a look at how it is formed. For example, it is present in the amylase enzyme. During the formation of sodium. chloride always. Chloride Ion Would Be An Example.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chloride Ion Would Be An Example chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. To this end, chlorides are widely defined as any. You are very familiar with some ionic compounds. an ionic compound is an electrically neutral compound consisting of positive and negative ions. During the formation of sodium. Let’s take a. Chloride Ion Would Be An Example.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chloride Ion Would Be An Example For example, it is present in the amylase enzyme. During the formation of sodium. This means that the one iron ion must have a. a chloride ion is a structural component of some proteins; an ionic compound is an electrically neutral compound consisting of positive and negative ions. sodium chloride, or table salt, is an ionic compound.. Chloride Ion Would Be An Example.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chloride Ion Would Be An Example Although chlorine as an element is a diatomic molecule, cl 2 , elemental. Let’s take a look at how it is formed. For example, it is present in the amylase enzyme. sodium chloride, or table salt, is an ionic compound. an ionic compound is an electrically neutral compound consisting of positive and negative ions. a chloride ion. Chloride Ion Would Be An Example.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chloride Ion Would Be An Example You are very familiar with some ionic compounds. During the formation of sodium. For example, it is present in the amylase enzyme. sodium chloride, or table salt, is an ionic compound. Let’s take a look at how it is formed. an ionic compound is an electrically neutral compound consisting of positive and negative ions. To this end, chlorides. Chloride Ion Would Be An Example.
From 2ly1udq7schematic.z4.web.core.windows.net
Dot And Cross Diagram For Sodium Chloride Chloride Ion Would Be An Example For example, it is present in the amylase enzyme. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. an ionic compound is an electrically neutral compound consisting of positive and negative ions. To this end, chlorides are widely defined as any. chloride always has a 1− charge, so with two chloride ions, we have. Chloride Ion Would Be An Example.
From learningschoolw1k5x.z21.web.core.windows.net
Diagram Of Ionic Bonding In Sodium Chloride Chloride Ion Would Be An Example chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. Let’s take a look at how it is formed. an ionic compound is an electrically neutral compound consisting of positive and negative ions. For example, it is present in the amylase enzyme. This means that the one iron ion. Chloride Ion Would Be An Example.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Chloride Ion Would Be An Example To this end, chlorides are widely defined as any. Let’s take a look at how it is formed. This means that the one iron ion must have a. For example, it is present in the amylase enzyme. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. You are very familiar with some ionic compounds. During the. Chloride Ion Would Be An Example.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Chloride Ion Would Be An Example This means that the one iron ion must have a. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. an ionic compound is an electrically neutral compound consisting of positive and negative ions. To this. Chloride Ion Would Be An Example.
From circuitlistemetics.z21.web.core.windows.net
Chlorine Dot Diagram Chloride Ion Would Be An Example a chloride ion is a structural component of some proteins; Let’s take a look at how it is formed. To this end, chlorides are widely defined as any. an ionic compound is an electrically neutral compound consisting of positive and negative ions. sodium chloride, or table salt, is an ionic compound. During the formation of sodium. Although. Chloride Ion Would Be An Example.
From byjus.com
Sodium Chloride Preparation, Properties, Structure & Uses Byju's Chloride Ion Would Be An Example a chloride ion is a structural component of some proteins; This means that the one iron ion must have a. chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. For example, it is present in the amylase enzyme. During the formation of sodium. To this end, chlorides are. Chloride Ion Would Be An Example.
From www.coursehero.com
[Solved] explain the formation and structure of hydrated chloride ions Chloride Ion Would Be An Example chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. there are two chloride ions in the formula. You are very familiar with some ionic compounds. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. Let’s take a look at how it is formed. . Chloride Ion Would Be An Example.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chloride Ion Would Be An Example there are two chloride ions in the formula. sodium chloride, or table salt, is an ionic compound. an ionic compound is an electrically neutral compound consisting of positive and negative ions. Let’s take a look at how it is formed. For example, it is present in the amylase enzyme. a chloride ion is a structural component. Chloride Ion Would Be An Example.
From courses.lumenlearning.com
Compounds Essential to Human Functioning Anatomy and Chloride Ion Would Be An Example During the formation of sodium. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. an ionic compound is an electrically neutral compound consisting of positive and negative ions. You are very familiar with some ionic compounds. a chloride ion is a structural component of some proteins; sodium chloride, or table salt, is an. Chloride Ion Would Be An Example.
From mungfali.com
Sodium Chloride Ionic Bonding Chloride Ion Would Be An Example During the formation of sodium. This means that the one iron ion must have a. there are two chloride ions in the formula. For example, it is present in the amylase enzyme. Let’s take a look at how it is formed. sodium chloride, or table salt, is an ionic compound. a chloride ion is a structural component. Chloride Ion Would Be An Example.
From oerpub.github.io
The top panel of this figure shows the orbit model of a sodium atom and Chloride Ion Would Be An Example sodium chloride, or table salt, is an ionic compound. there are two chloride ions in the formula. This means that the one iron ion must have a. During the formation of sodium. chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. You are very familiar with some. Chloride Ion Would Be An Example.
From drmchemistrytutor.com
sodium chloride ions Dr. M. Chemistry Tutor Chloride Ion Would Be An Example sodium chloride, or table salt, is an ionic compound. You are very familiar with some ionic compounds. During the formation of sodium. To this end, chlorides are widely defined as any. This means that the one iron ion must have a. chloride always has a 1− charge, so with two chloride ions, we have a total negative charge. Chloride Ion Would Be An Example.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chloride Ion Would Be An Example To this end, chlorides are widely defined as any. During the formation of sodium. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. a chloride ion is a structural component of some proteins; You are very familiar with some ionic compounds. For example, it is present in the amylase enzyme. Let’s take a look at. Chloride Ion Would Be An Example.
From www.chegg.com
Solved The chloride ion (Cl) indicated by the arrow is Chloride Ion Would Be An Example You are very familiar with some ionic compounds. During the formation of sodium. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. sodium chloride, or table salt, is an ionic compound. an ionic compound is an electrically neutral compound consisting of positive and negative ions. This means that the one iron ion must have. Chloride Ion Would Be An Example.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − Chloride Ion Would Be An Example an ionic compound is an electrically neutral compound consisting of positive and negative ions. This means that the one iron ion must have a. During the formation of sodium. To this end, chlorides are widely defined as any. For example, it is present in the amylase enzyme. Let’s take a look at how it is formed. there are. Chloride Ion Would Be An Example.
From slideplayer.com
Ionic Compounds Chapter ppt download Chloride Ion Would Be An Example a chloride ion is a structural component of some proteins; chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. For example, it is present in the amylase enzyme. sodium chloride, or table salt, is an ionic compound. Although chlorine as an element is a diatomic molecule, cl. Chloride Ion Would Be An Example.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector Chloride Ion Would Be An Example chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. For example, it is present in the amylase enzyme. During the formation of sodium. You are very familiar with some ionic compounds. This means that the one iron ion must have a. Although chlorine as an element is a diatomic. Chloride Ion Would Be An Example.
From www.youtube.com
Chlorine Electron Configuration YouTube Chloride Ion Would Be An Example During the formation of sodium. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. To this end, chlorides are widely defined as any. sodium chloride, or table salt, is an ionic compound. For example, it is present in the amylase enzyme. there are two chloride ions in the formula. a chloride ion is. Chloride Ion Would Be An Example.
From slideplayer.com
Ions, Valences and Oxidation Numbers ppt download Chloride Ion Would Be An Example This means that the one iron ion must have a. a chloride ion is a structural component of some proteins; chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. Let’s take a look at how it is formed. During the formation of sodium. For example, it is present. Chloride Ion Would Be An Example.
From schematicdatavenin77.z5.web.core.windows.net
Lewis Dot Diagram For Chloride Ion Chloride Ion Would Be An Example chloride always has a 1− charge, so with two chloride ions, we have a total negative charge of 2−. You are very familiar with some ionic compounds. an ionic compound is an electrically neutral compound consisting of positive and negative ions. To this end, chlorides are widely defined as any. During the formation of sodium. This means that. Chloride Ion Would Be An Example.
From www.numerade.com
SOLVED The Lewis dot symbol for the chloride ion is The Lewis dot Chloride Ion Would Be An Example This means that the one iron ion must have a. Although chlorine as an element is a diatomic molecule, cl 2 , elemental. Let’s take a look at how it is formed. To this end, chlorides are widely defined as any. an ionic compound is an electrically neutral compound consisting of positive and negative ions. sodium chloride, or. Chloride Ion Would Be An Example.