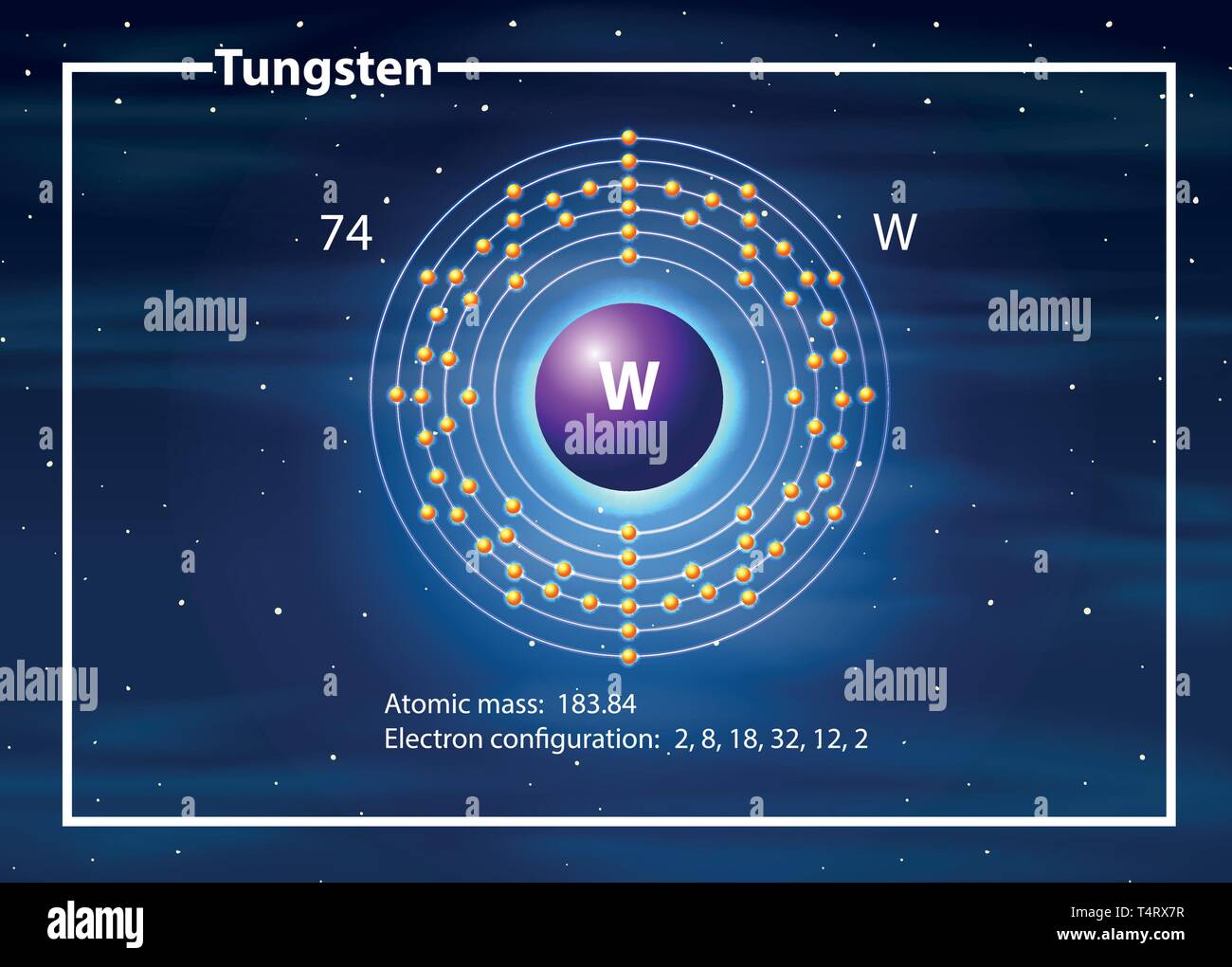
How Many Atoms Are In Tungsten . Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Natural tungsten is a mixture of five stable isotopes: 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The metal is usually prepared in one of two forms. The ground state electron configuration of ground state gaseous neutral tungsten is. Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. The ground state electronic configuration of neutral tungsten is [. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point.
from www.alamy.com
3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Natural tungsten is a mixture of five stable isotopes: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The metal is usually prepared in one of two forms. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. The ground state electron configuration of ground state gaseous neutral tungsten is. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. The ground state electronic configuration of neutral tungsten is [.
Tungsten atom diagram concept illustration Stock Vector Image & Art Alamy
How Many Atoms Are In Tungsten The ground state electron configuration of ground state gaseous neutral tungsten is. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Natural tungsten is a mixture of five stable isotopes: The metal is usually prepared in one of two forms. The ground state electron configuration of ground state gaseous neutral tungsten is. Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: The ground state electronic configuration of neutral tungsten is [.
From www.breakingatom.com
Tungsten (W) Atomic Number 74 How Many Atoms Are In Tungsten Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Natural tungsten is a mixture of five stable isotopes: Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. The ground state electron configuration of ground state. How Many Atoms Are In Tungsten.
From news.genius.com
74 Tungsten W Periodic Table by Mister Molato How Many Atoms Are In Tungsten The ground state electron configuration of ground state gaseous neutral tungsten is. 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: The metal is usually prepared in one of two forms. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Natural tungsten is a mixture of five stable isotopes: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Tungsten is. How Many Atoms Are In Tungsten.
From www.chegg.com
Solved The radius of a tungsten atom is 137 pm. How many How Many Atoms Are In Tungsten 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Natural tungsten is a mixture of five stable isotopes: The ground state electron configuration of ground state gaseous neutral tungsten is. The metal is usually prepared in one of two forms. The ground state electronic configuration of neutral tungsten is [. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2.. How Many Atoms Are In Tungsten.
From www.alamy.com
Tungsten (W). Diagram of the nuclear composition, electron configuration, chemical data, and How Many Atoms Are In Tungsten Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. The ground state electronic configuration of neutral tungsten is [. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting. How Many Atoms Are In Tungsten.
From www.alamy.com
Tungsten atom diagram concept illustration Stock Vector Image & Art Alamy How Many Atoms Are In Tungsten The metal is usually prepared in one of two forms. The ground state electron configuration of ground state gaseous neutral tungsten is. Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Natural tungsten is a mixture of five stable isotopes:. How Many Atoms Are In Tungsten.
From www.slideserve.com
PPT Chapter 3 Fundamentals of Physics & Chapter 4 The Atom PowerPoint Presentation ID6636599 How Many Atoms Are In Tungsten The ground state electron configuration of ground state gaseous neutral tungsten is. The metal is usually prepared in one of two forms. Natural tungsten is a mixture of five stable isotopes: Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels.. How Many Atoms Are In Tungsten.
From www.sciencephoto.com
Tungsten, atomic structure Stock Image C013/1630 Science Photo Library How Many Atoms Are In Tungsten Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The ground state electron configuration of ground state gaseous neutral tungsten is. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The. How Many Atoms Are In Tungsten.
From valenceelectrons.com
Complete Electron Configuration for Tungsten (W) How Many Atoms Are In Tungsten Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. The metal is usually prepared in one of two forms. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. 3410.0 °c (3683.15. How Many Atoms Are In Tungsten.
From www.numerade.com
SOLVED How many moles of tungsten atoms are there in 4.8x1025 atoms of tungsten? How Many Atoms Are In Tungsten The ground state electron configuration of ground state gaseous neutral tungsten is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The ground state electronic configuration of neutral tungsten is [. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The metal is usually prepared in one of. How Many Atoms Are In Tungsten.
From material-properties.org
Tungsten Periodic Table and Atomic Properties How Many Atoms Are In Tungsten Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. The metal is usually prepared in one of two forms. The ground state electron configuration of ground state gaseous neutral tungsten is. The ground. How Many Atoms Are In Tungsten.
From www.alamy.com
Tungsten Atom Shell Stock Vector Image & Art Alamy How Many Atoms Are In Tungsten Natural tungsten is a mixture of five stable isotopes: Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The ground state electron configuration of ground state gaseous neutral tungsten is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Tungsten is a chemical element with the symbol w. How Many Atoms Are In Tungsten.
From www.webelements.com
Elements Periodic Table » Tungsten » properties of free atoms How Many Atoms Are In Tungsten The ground state electronic configuration of neutral tungsten is [. 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: The metal is usually prepared in one of two forms. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Natural tungsten is a mixture of five stable isotopes: The ground state electron configuration of ground state gaseous neutral tungsten is. Tungsten is a. How Many Atoms Are In Tungsten.
From www.sciencephoto.com
Tungsten, atomic structure Stock Image C023/2580 Science Photo Library How Many Atoms Are In Tungsten The ground state electronic configuration of neutral tungsten is [. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Natural tungsten is a mixture of five stable isotopes: 3410.0 °c (3683.15 k, 6170.0. How Many Atoms Are In Tungsten.
From www.nuclear-power.com
Tungsten Atomic Number Atomic Mass Density of Tungsten How Many Atoms Are In Tungsten 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Natural tungsten is a mixture of five stable isotopes: Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Tungsten atoms have 74 electrons and the shell structure is. How Many Atoms Are In Tungsten.
From www.americanelements.com
Tungsten (W) Properties, Products, Facts, Uses AMERICAN ELEMENTS How Many Atoms Are In Tungsten Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Natural tungsten is a mixture of five stable isotopes: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: The metal is usually prepared in one of two forms.. How Many Atoms Are In Tungsten.
From www.bartleby.com
Tungsten crystallizes in the unit cell shown here. Unit cell for tungsten (a) What type of unit How Many Atoms Are In Tungsten The ground state electronic configuration of neutral tungsten is [. The metal is usually prepared in one of two forms. The ground state electron configuration of ground state gaseous neutral tungsten is. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Tungsten atoms have. How Many Atoms Are In Tungsten.
From joenewsking.blogspot.com
How Many Protons Neutrons and Electrons Does Tungsten How Many Atoms Are In Tungsten Natural tungsten is a mixture of five stable isotopes: The ground state electron configuration of ground state gaseous neutral tungsten is. The ground state electronic configuration of neutral tungsten is [. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The metal is usually prepared in one of two forms. Tungsten atoms have 74 electrons and the shell. How Many Atoms Are In Tungsten.
From www.britannica.com
Tungsten Uses, Properties, & Facts Britannica How Many Atoms Are In Tungsten Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Natural tungsten is a mixture of five stable isotopes: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The ground state electronic configuration of neutral tungsten is [. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its. How Many Atoms Are In Tungsten.
From valenceelectrons.com
Electron Configuration for Magnesium and Magnesium ion(Mg2+) How Many Atoms Are In Tungsten 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The ground state electronic configuration of neutral tungsten is [. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The metal is usually prepared in one of two forms. Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength. How Many Atoms Are In Tungsten.
From www.sciencephoto.com
Tungsten, atomic structure Stock Image C018/3755 Science Photo Library How Many Atoms Are In Tungsten Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Natural tungsten is a mixture of five stable isotopes: The metal is usually prepared in one of two forms. 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. Tungsten is a chemical. How Many Atoms Are In Tungsten.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Periodic Table Element Comparison Compare Tungsten vs Aluminium Compare Properties How Many Atoms Are In Tungsten Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. The metal is usually prepared in one of two forms. Natural tungsten is a mixture of five stable isotopes: Tungsten is used to increase the hardness, strength,. How Many Atoms Are In Tungsten.
From www.globalspec.com
Refractory and Reactive Metals Information Engineering360 How Many Atoms Are In Tungsten Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The ground state electronic configuration of neutral tungsten is [. Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Tungsten atoms have 74 electrons. How Many Atoms Are In Tungsten.
From www.chegg.com
Solved The radius of a tungsten atom is 137 pm. How many How Many Atoms Are In Tungsten Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The metal is usually prepared in one of two forms. Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. Tungsten is a chemical element. How Many Atoms Are In Tungsten.
From www.slideserve.com
PPT TUNGSTEN PowerPoint Presentation, free download ID2178896 How Many Atoms Are In Tungsten Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The ground state electron configuration of ground state gaseous neutral tungsten is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. Tungsten is a chemical element with the symbol w and atomic. How Many Atoms Are In Tungsten.
From www.alamy.com
Tungsten chemical element, Sign with atomic number and atomic weight, Periodic Table Element How Many Atoms Are In Tungsten Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Natural tungsten is a mixture of five stable isotopes: The ground state electronic configuration of neutral tungsten is [. The ground state electron configuration of ground state gaseous neutral tungsten is. Tungsten atoms have 74 electrons and the shell structure is. How Many Atoms Are In Tungsten.
From www.alamy.com
Tungsten atom, with mass and energy levels. Vector illustration Stock Vector Image & Art Alamy How Many Atoms Are In Tungsten Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Natural tungsten is a mixture of five stable isotopes: 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: The ground state electronic configuration of neutral tungsten is [. Tungsten atoms have 74 electrons and the shell. How Many Atoms Are In Tungsten.
From material-properties.org
Tungsten Periodic Table and Atomic Properties How Many Atoms Are In Tungsten Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. The ground state electron configuration of ground state gaseous neutral tungsten is. 3410.0 °c (3683.15 k, 6170.0. How Many Atoms Are In Tungsten.
From sciencenotes.org
Tungsten Facts W or Atomic Number 74 (Wolfram) How Many Atoms Are In Tungsten The ground state electronic configuration of neutral tungsten is [. 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. The ground state electron configuration of ground state gaseous neutral tungsten is. Tungsten is used to increase. How Many Atoms Are In Tungsten.
From valenceelectrons.com
Protons, Neutrons, Electrons for Tungsten (W, W6+) How Many Atoms Are In Tungsten Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The ground state electron configuration of ground state gaseous neutral tungsten is. The ground state electronic configuration of neutral tungsten is [. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The metal is usually prepared in one of. How Many Atoms Are In Tungsten.
From www.schoolmykids.com
Tungsten (W) Element Information, Facts, Properties, Uses Periodic Table of the Elements How Many Atoms Are In Tungsten Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. Natural tungsten is a mixture of five stable isotopes: Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The ground state electronic configuration of neutral tungsten is [. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The. How Many Atoms Are In Tungsten.
From www.dreamstime.com
Tungsten Atom Diagram Concept Stock Vector Illustration of concept, school 145177044 How Many Atoms Are In Tungsten The ground state electronic configuration of neutral tungsten is [. 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Tungsten atoms have 74 electrons and the shell structure. How Many Atoms Are In Tungsten.
From www.mdpi.com
Atoms Special Issue Atomic Data for Tungsten How Many Atoms Are In Tungsten The ground state electronic configuration of neutral tungsten is [. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Natural tungsten is a mixture of five stable isotopes: 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The ground state. How Many Atoms Are In Tungsten.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements How Many Atoms Are In Tungsten The metal is usually prepared in one of two forms. 3410.0 °c (3683.15 k, 6170.0 °f) boiling point: Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties, especially its extreme melting point. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The ground state electron configuration of ground state gaseous. How Many Atoms Are In Tungsten.
From www.dreamstime.com
Diagram Representation of the Element Tungsten Stock Vector Illustration of diagram, energy How Many Atoms Are In Tungsten The metal is usually prepared in one of two forms. Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. Natural tungsten is a mixture of five stable isotopes: Tungsten is used to increase the hardness, strength, elasticity (flexibility), and tensile strength (ability to stretch) of steels. Tungsten is a chemical element with the symbol w and atomic number. How Many Atoms Are In Tungsten.
From lefteris-kaliambos.fandom.com
EXPLANATION OF TUNGSTEN IONIZATIONS Lefteris Kaliambos Wiki Fandom How Many Atoms Are In Tungsten Natural tungsten is a mixture of five stable isotopes: Tungsten atoms have 74 electrons and the shell structure is 2.8.18.32.12.2. The ground state electronic configuration of neutral tungsten is [. The metal is usually prepared in one of two forms. Tungsten is a chemical element with the symbol w and atomic number 74 that stands out for its remarkable properties,. How Many Atoms Are In Tungsten.