Spectrophotometer Calculation Absorbance . Find the molar absorptivity units, the formula, and the applications. The amount of light that is absorbed by the sample is measured. Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. Understand the concepts of absorbance, beer's law, spectra and calibration. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. See the equation, the graph, and the video example. In general, a light shines through a cuvette filled with a sample. Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. Learn how to use beer's law to calculate the absorbance and concentration of solutions. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer.
from pro-analytics.net
Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. Learn how to use beer's law to calculate the absorbance and concentration of solutions. Find the molar absorptivity units, the formula, and the applications. In general, a light shines through a cuvette filled with a sample. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. The amount of light that is absorbed by the sample is measured. Understand the concepts of absorbance, beer's law, spectra and calibration. See the equation, the graph, and the video example. Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value.
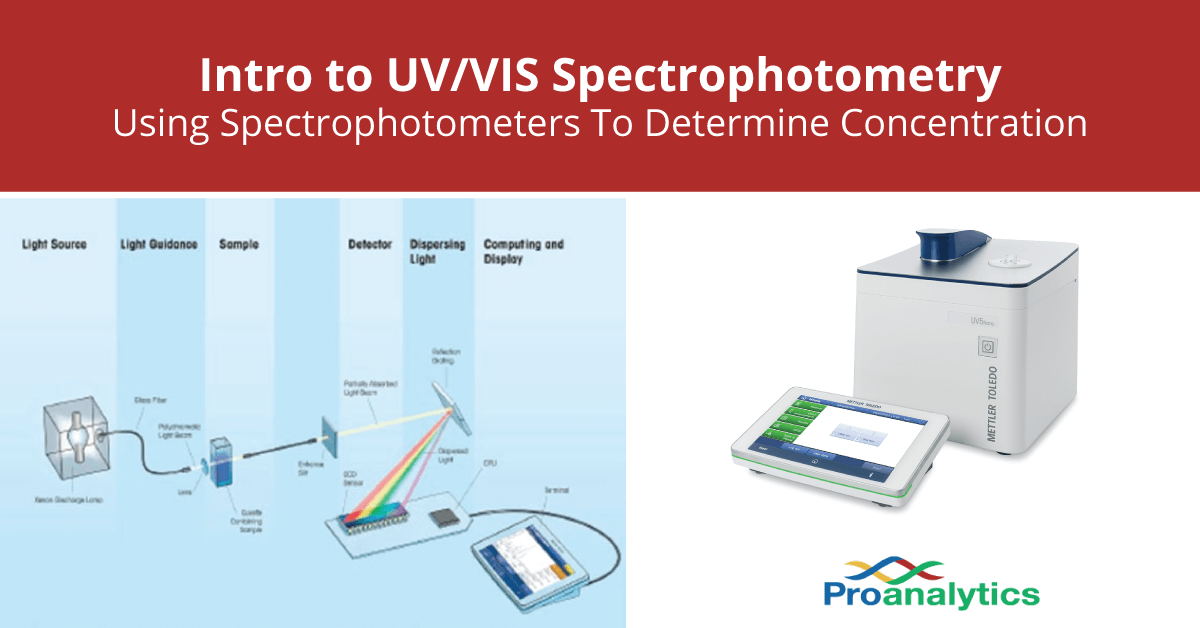
Using Spectrophotometer To Determine Concentration (UV/VIS)
Spectrophotometer Calculation Absorbance Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. Understand the concepts of absorbance, beer's law, spectra and calibration. In general, a light shines through a cuvette filled with a sample. See the equation, the graph, and the video example. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Learn how to use beer's law to calculate the absorbance and concentration of solutions. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. The amount of light that is absorbed by the sample is measured. Find the molar absorptivity units, the formula, and the applications. Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value.
From www.chegg.com
Solved Spectrophotometers are used to measure the absorbance Spectrophotometer Calculation Absorbance Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. Understand the concepts of absorbance, beer's law, spectra and calibration. Learn how to use beer's law to calculate the absorbance and concentration of solutions. See the equation, the graph, and the video example. In general, a light shines through a cuvette filled with a sample. The. Spectrophotometer Calculation Absorbance.
From www.ssi.shimadzu.com
How does a spectrophotometer measure a sample? Shimadzu Scientific Instruments Spectrophotometer Calculation Absorbance Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. In general, a light shines through a cuvette filled with a sample. Find the molar absorptivity units, the formula, and the applications. See the equation, the graph, and the. Spectrophotometer Calculation Absorbance.
From uniapaclisbon2018.com
What Units Are Used To Measure Absorbance On A Spectrophotometer Spectrophotometer Calculation Absorbance Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Find the molar absorptivity units, the formula, and the applications. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits.. Spectrophotometer Calculation Absorbance.
From shaniyaukung.blogspot.com
What Is Absorbance In Spectrophotometer Spectrophotometer Calculation Absorbance Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. Find the molar absorptivity units, the formula, and the applications. In general, a light shines through a cuvette filled with a sample. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Learn how to. Spectrophotometer Calculation Absorbance.
From websites.umich.edu
Chem 125 Experiment II Spectrophotometer Calculation Absorbance Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. Find the molar absorptivity units, the formula, and the applications. In general, a light shines through a cuvette filled with a sample. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Understand the concepts. Spectrophotometer Calculation Absorbance.
From inds.co.uk
SpectroVis Plus Spectrophotometer Spectrophotometer Calculation Absorbance A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Find the molar absorptivity units, the formula, and the applications. See the equation, the graph, and the video example. Understand the concepts of absorbance, beer's law, spectra and calibration. The amount of light that is absorbed by the sample is measured. In general,. Spectrophotometer Calculation Absorbance.
From www.linquip.com
Beginners Guide What Is a Spectrophotometer Industrial Manufacturing Blog linquip Spectrophotometer Calculation Absorbance Understand the concepts of absorbance, beer's law, spectra and calibration. Find the molar absorptivity units, the formula, and the applications. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Learn how to use beer's law to calculate the absorbance and concentration of solutions. See the equation, the graph, and the video example.. Spectrophotometer Calculation Absorbance.
From www.researchgate.net
Standard calibration curve of Dglucose (absorbance measured at 489 nm) Download Scientific Spectrophotometer Calculation Absorbance See the equation, the graph, and the video example. Learn how to use beer's law to calculate the absorbance and concentration of solutions. Understand the concepts of absorbance, beer's law, spectra and calibration. The amount of light that is absorbed by the sample is measured. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in. Spectrophotometer Calculation Absorbance.
From www.youtube.com
how to measure optical density with spectrophotometer YouTube Spectrophotometer Calculation Absorbance A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. The amount of light that is absorbed by the sample is measured. See the equation, the graph, and the video example. Understand the concepts of absorbance, beer's law, spectra. Spectrophotometer Calculation Absorbance.
From pro-analytics.net
Using Spectrophotometer To Determine Concentration (UV/VIS) Spectrophotometer Calculation Absorbance Find the molar absorptivity units, the formula, and the applications. See the equation, the graph, and the video example. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Understand the concepts of absorbance, beer's law, spectra and calibration. The amount of light that is absorbed by the sample is measured. Learn how. Spectrophotometer Calculation Absorbance.
From www.researchgate.net
How to calculate the assay in UVVis spectrophotometer, if specific absorbance knows? ResearchGate Spectrophotometer Calculation Absorbance Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. The amount of light that is absorbed by the sample is measured. See the equation, the graph, and the video example. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to use beer's law. Spectrophotometer Calculation Absorbance.
From www.researchgate.net
The relationship between absorbance measured on a spectrophotometer... Download Scientific Diagram Spectrophotometer Calculation Absorbance In general, a light shines through a cuvette filled with a sample. See the equation, the graph, and the video example. Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. Understand the concepts of absorbance, beer's law, spectra and calibration. Learn how to use beer's law to find the concentration of an unknown sample from. Spectrophotometer Calculation Absorbance.
From mungfali.com
Standard Curve Graph Spectrophotometer Calculation Absorbance The amount of light that is absorbed by the sample is measured. Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to use beer's law to find the concentration of an unknown sample from. Spectrophotometer Calculation Absorbance.
From www.numerade.com
SOLVED UV Spectrophotometer Test Tube Mol of Copper sulfate (Iol) Volume Concentration Spectrophotometer Calculation Absorbance See the equation, the graph, and the video example. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Understand the concepts of absorbance, beer's law, spectra and calibration. The amount of light that. Spectrophotometer Calculation Absorbance.
From conductscience.com
Spectrophotometer Conduct Science Spectrophotometer Calculation Absorbance See the equation, the graph, and the video example. In general, a light shines through a cuvette filled with a sample. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to use beer's law to calculate the absorbance and concentration of solutions. Understand the concepts of absorbance, beer's. Spectrophotometer Calculation Absorbance.
From schematicgonzina4e.z13.web.core.windows.net
Atomic Absorption Spectroscopy Schematic Diagram Spectrophotometer Calculation Absorbance See the equation, the graph, and the video example. Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. Learn how to use beer's law to calculate the absorbance and concentration of solutions. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Convert spectrophotometer. Spectrophotometer Calculation Absorbance.
From www.semanticscholar.org
[PDF] Introduction to the Spectrophotometer Wavelength , Absorbance , and Concentration In Spectrophotometer Calculation Absorbance Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. Understand the concepts of absorbance, beer's law, spectra and calibration. Learn how to use beer's law to calculate the absorbance and concentration of solutions. Find the molar absorptivity units, the formula, and the applications. A spectrophotometer is used to determine the concentration of certain compounds, such. Spectrophotometer Calculation Absorbance.
From www.youtube.com
Atomic Absorption Spectrometer (AAS) YouTube Spectrophotometer Calculation Absorbance In general, a light shines through a cuvette filled with a sample. See the equation, the graph, and the video example. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. The amount of light that is absorbed by the sample is measured. Find the molar absorptivity units, the formula, and the applications.. Spectrophotometer Calculation Absorbance.
From www.researchgate.net
(a) Absorbance spectra collected with the microscopecoupled... Download Scientific Diagram Spectrophotometer Calculation Absorbance In general, a light shines through a cuvette filled with a sample. Understand the concepts of absorbance, beer's law, spectra and calibration. The amount of light that is absorbed by the sample is measured. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. A spectrophotometer is used to determine the. Spectrophotometer Calculation Absorbance.
From scienceinfo.com
Atomic Absorption Spectrophotometry Principle, Parts, Uses Spectrophotometer Calculation Absorbance A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Understand the concepts of absorbance, beer's law, spectra and calibration. Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. In general, a light shines through a cuvette filled with a sample. Find the molar. Spectrophotometer Calculation Absorbance.
From www.researchgate.net
Uvvis Absorbance spectra of as synthesized ZnO NPs in the solution... Download Scientific Diagram Spectrophotometer Calculation Absorbance Find the molar absorptivity units, the formula, and the applications. Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. See the equation, the graph, and the video example. Learn how to use beer's law to calculate the absorbance and concentration of solutions. Understand the concepts of absorbance, beer's law, spectra and. Spectrophotometer Calculation Absorbance.
From gionlujil.blob.core.windows.net
Does Spectrophotometer Calculate Absorbance at Ronald Gonzalez blog Spectrophotometer Calculation Absorbance Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. The amount of light that is absorbed by the sample is measured. See the equation, the graph, and the video example. Find the molar absorptivity units, the formula, and the applications. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in. Spectrophotometer Calculation Absorbance.
From www.researchgate.net
Absorbance spectra of UVVis spectrophotometer and band gap calculation... Download Scientific Spectrophotometer Calculation Absorbance Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. In general, a light shines through a cuvette filled with a sample. Understand the concepts of absorbance, beer's law, spectra and calibration. Learn how to use beer's law to calculate the absorbance and concentration of solutions. Convert spectrophotometer absorbance reading to. Spectrophotometer Calculation Absorbance.
From www.scribd.com
Calibration of UV Spectrophotometer Absorbance Spectrum Spectrophotometer Calculation Absorbance The amount of light that is absorbed by the sample is measured. Learn how to use beer's law to calculate the absorbance and concentration of solutions. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Find the molar absorptivity units, the formula, and the applications. Convert spectrophotometer absorbance reading to. Spectrophotometer Calculation Absorbance.
From bloodbankdepot.com
absorbance spectrophotometer wave length reader Spectrophotometer Calculation Absorbance Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. See the equation, the graph, and the video example. Find. Spectrophotometer Calculation Absorbance.
From www.vernier.com
Decoding Your Absorbance Readings Vernier Spectrophotometer Calculation Absorbance Learn how to use beer's law to calculate the absorbance and concentration of solutions. Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. Understand the concepts of absorbance, beer's law, spectra and calibration. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Find the molar absorptivity units,. Spectrophotometer Calculation Absorbance.
From ibsen.com
How to Obtain a Large Absorbance Range Ibsen Photonics Spectrophotometer Calculation Absorbance Find the molar absorptivity units, the formula, and the applications. See the equation, the graph, and the video example. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. Learn how to measure the amount. Spectrophotometer Calculation Absorbance.
From www.youtube.com
Atomic Absorbance Spectrophotometer YouTube Spectrophotometer Calculation Absorbance In general, a light shines through a cuvette filled with a sample. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. The amount of light that is absorbed by the sample is measured. Find the molar absorptivity units, the formula, and the applications. See the equation, the graph, and the video example.. Spectrophotometer Calculation Absorbance.
From www.researchgate.net
Calibration of spectrophotometer by measuring absorbance of CuSO4... Download Scientific Diagram Spectrophotometer Calculation Absorbance A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Find the molar absorptivity units, the formula, and the applications. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to use beer's law to calculate the absorbance and concentration of solutions.. Spectrophotometer Calculation Absorbance.
From www.youtube.com
Absorption Readings from Spectrometer (Spectrometry) YouTube Spectrophotometer Calculation Absorbance Understand the concepts of absorbance, beer's law, spectra and calibration. Find the molar absorptivity units, the formula, and the applications. Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. In general, a light shines. Spectrophotometer Calculation Absorbance.
From studyparamnesia.z21.web.core.windows.net
How To Find Absorbance Coefficient Spectrophotometer Calculation Absorbance Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. Understand the concepts of absorbance, beer's law, spectra and calibration. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. See the equation, the graph, and the video example. Learn how to use beer's law. Spectrophotometer Calculation Absorbance.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download ID4942160 Spectrophotometer Calculation Absorbance Learn how to use beer's law to calculate the absorbance and concentration of solutions. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. A spectrophotometer is used to determine the concentration of certain compounds, such as protein, in a solution. Find the molar absorptivity units, the formula, and the applications.. Spectrophotometer Calculation Absorbance.
From slideplayer.com
Lab1 A VISIBLE ABSORPTION SPECTROMETER ppt download Spectrophotometer Calculation Absorbance Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. In general, a light shines through a cuvette filled with a sample. Understand the concepts of absorbance, beer's law, spectra and calibration. See the equation, the graph, and the video example. Learn how to measure the amount of light absorbed by a sample at a specific. Spectrophotometer Calculation Absorbance.
From www.scribd.com
Spectrophotometer PDF Spectrophotometry Absorbance Spectrophotometer Calculation Absorbance Learn how to use beer's law to calculate the absorbance and concentration of solutions. Learn how to use beer's law to find the concentration of an unknown sample from its absorbance value. In general, a light shines through a cuvette filled with a sample. Convert spectrophotometer absorbance reading to test result (ppm) for all chemetrics instrumental test kits. The amount. Spectrophotometer Calculation Absorbance.
From definitionhjo.blogspot.com
Definition Of Absorbance In Chemistry DEFINITION HJO Spectrophotometer Calculation Absorbance Find the molar absorptivity units, the formula, and the applications. The amount of light that is absorbed by the sample is measured. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. In general, a light shines through a cuvette filled with a sample. A spectrophotometer is used to determine the. Spectrophotometer Calculation Absorbance.