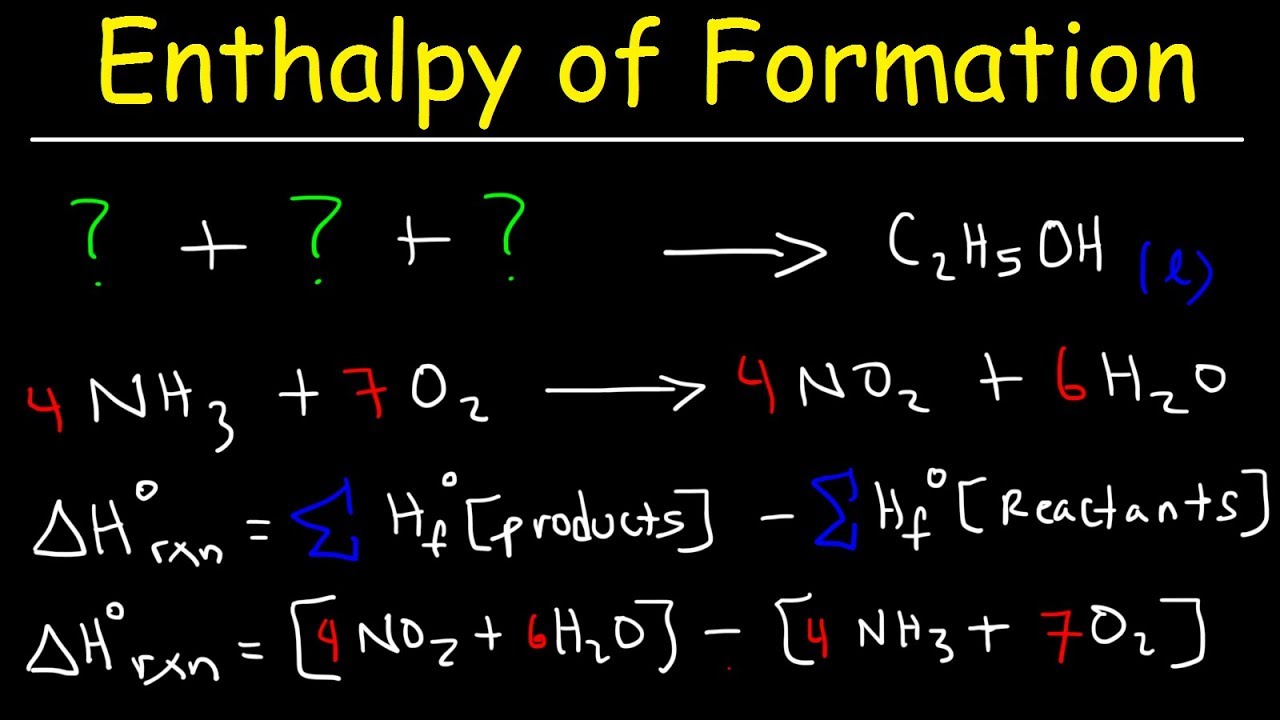
Standard Heat Of Formation Formula . By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. The standard state heat of formation for the elemental form of each atom is zero. The standard state of an element can be identified in. The elemental form of each atom is that with the lowest enthalpy in the standard state. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. The nought superscript means standard state. The standard enthalpy (heat) of reaction is given by δh orxn. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free.
from lessonsybil.z5.web.core.windows.net
The standard enthalpy (heat) of reaction is given by δh orxn. The standard state heat of formation for the elemental form of each atom is zero. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The elemental form of each atom is that with the lowest enthalpy in the standard state. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. The nought superscript means standard state. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. The standard state of an element can be identified in.
Heat Of Formation Equations
Standard Heat Of Formation Formula The nought superscript means standard state. The standard enthalpy (heat) of reaction is given by δh orxn. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. The nought superscript means standard state. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state of an element can be identified in. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard state heat of formation for the elemental form of each atom is zero.
From www.researchgate.net
Coefficient values for heat capacity equation and Standard Enthalpy of... Download Scientific Standard Heat Of Formation Formula The standard state heat of formation for the elemental form of each atom is zero. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. The elemental form of each atom is that with the lowest enthalpy in the standard state. By definition, the standard enthalpy of formation of. Standard Heat Of Formation Formula.
From www.msjchem.com
Topic 5 Energetics MSJChem Tutorial videos for IB Chemistry Standard Heat Of Formation Formula This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. The standard state heat of formation for the elemental form of each atom is zero. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in. Standard Heat Of Formation Formula.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Heat Of Formation Formula 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy (heat) of reaction is given by δh orxn. The. Standard Heat Of Formation Formula.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download ID5759361 Standard Heat Of Formation Formula To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies. Standard Heat Of Formation Formula.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Heat Of Formation Formula The nought superscript means standard state. The standard enthalpy (heat) of reaction is given by δh orxn. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. This equation essentially states that the standard enthalpy change of. Standard Heat Of Formation Formula.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation, free download ID2964088 Standard Heat Of Formation Formula A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for.. Standard Heat Of Formation Formula.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Heat Of Formation Formula The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy (heat) of reaction is given by δh orxn. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. To. Standard Heat Of Formation Formula.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Formula A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state heat of formation for the elemental form of each atom is zero. This equation essentially states that the standard enthalpy change of formation is. Standard Heat Of Formation Formula.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Heat Of Formation Formula The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy (heat) of reaction is given by δh orxn. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. 193 rows the standard enthalpy change of. Standard Heat Of Formation Formula.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g)+O2(g) > 2H2O(I) Standard Heat Of Formation Formula This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. By definition, the standard enthalpy of formation of an element in its most stable form is. Standard Heat Of Formation Formula.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Of Formation Formula The standard state of an element can be identified in. The standard enthalpy (heat) of reaction is given by δh orxn. The elemental form of each atom is that with the lowest enthalpy in the standard state. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. This equation. Standard Heat Of Formation Formula.
From www.youtube.com
Standard Enthalpy of Formation for Benzene YouTube Standard Heat Of Formation Formula The standard state of an element can be identified in. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of. Standard Heat Of Formation Formula.
From narodnatribuna.info
Enthalpy Equation Standard Heat Of Formation Formula 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. The standard state of an element can be identified in. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. This equation. Standard Heat Of Formation Formula.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube Standard Heat Of Formation Formula A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for.. Standard Heat Of Formation Formula.
From printablelibsnivel.z21.web.core.windows.net
Heats Of Formation Equation Standard Heat Of Formation Formula To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. The nought superscript means standard state. The elemental form of each atom is that with the lowest enthalpy in. Standard Heat Of Formation Formula.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Heat Of Formation Formula A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy (heat) of reaction is given by δh orxn. By. Standard Heat Of Formation Formula.
From slideplayer.com
Standard Heat of Formation ppt download Standard Heat Of Formation Formula The nought superscript means standard state. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. The standard enthalpy (heat) of reaction is given by δh orxn. The elemental. Standard Heat Of Formation Formula.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Heat Of Formation Formula 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. The nought superscript means standard state. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free.. Standard Heat Of Formation Formula.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Heat Of Formation Formula A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. By definition, the standard enthalpy of. Standard Heat Of Formation Formula.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2841621 Standard Heat Of Formation Formula This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. To calculate the standard enthalpy of formation of a. Standard Heat Of Formation Formula.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Heat Of Formation Formula The nought superscript means standard state. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free.. Standard Heat Of Formation Formula.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation, free download ID2964088 Standard Heat Of Formation Formula The elemental form of each atom is that with the lowest enthalpy in the standard state. The nought superscript means standard state. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard state heat of formation for the elemental form of. Standard Heat Of Formation Formula.
From www.youtube.com
Writing Equations for Standard Enthalpy of Formation Examples YouTube Standard Heat Of Formation Formula A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state of an element can be identified in. The elemental form of each atom is that with the lowest enthalpy in the standard state. The. Standard Heat Of Formation Formula.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Appendix I to calculate the Standard Heat Of Formation Formula This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy (heat) of reaction is given by δh orxn. The nought superscript means standard state. 193 rows the. Standard Heat Of Formation Formula.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Heat Of Formation Formula 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy (heat) of reaction. Standard Heat Of Formation Formula.
From mungfali.com
Standard Enthalpy Change Equation Standard Heat Of Formation Formula The standard state of an element can be identified in. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. This equation essentially states that the standard enthalpy change. Standard Heat Of Formation Formula.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Heat Of Formation Formula The standard enthalpy (heat) of reaction is given by δh orxn. The elemental form of each atom is that with the lowest enthalpy in the standard state. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. The standard state heat of formation for the elemental form of each atom. Standard Heat Of Formation Formula.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Presentation ID3181539 Standard Heat Of Formation Formula A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. To calculate the standard enthalpy of. Standard Heat Of Formation Formula.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Heat Of Formation Formula The nought superscript means standard state. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state heat of formation for the elemental form of each atom is zero. 193 rows the standard enthalpy change. Standard Heat Of Formation Formula.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib Standard Heat Of Formation Formula The standard state of an element can be identified in. The elemental form of each atom is that with the lowest enthalpy in the standard state. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. This equation essentially states that the standard. Standard Heat Of Formation Formula.
From lessonsybil.z5.web.core.windows.net
Heat Of Formation Equations Standard Heat Of Formation Formula The standard enthalpy (heat) of reaction is given by δh orxn. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The nought superscript means standard state. This equation essentially states that the standard enthalpy change of. Standard Heat Of Formation Formula.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 Standard Heat Of Formation Formula 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. The nought superscript means standard state. This equation essentially states that the standard enthalpy change of formation is equal. Standard Heat Of Formation Formula.
From slideplayer.com
Standard Heat of Formation ppt download Standard Heat Of Formation Formula The nought superscript means standard state. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of. Standard Heat Of Formation Formula.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Heat Of Formation Formula To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero. Standard Heat Of Formation Formula.
From www.slideserve.com
PPT Ch. 15 Energy and Chemical Change PowerPoint Presentation, free download ID6309430 Standard Heat Of Formation Formula The elemental form of each atom is that with the lowest enthalpy in the standard state. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. The standard state heat of formation for the elemental form of each atom is zero. A standard enthalpy of formation δ h f. Standard Heat Of Formation Formula.