A Buffer Biology Quizlet . When there are too many h + ions, a buffer. the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. buffers are the key. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and donate h+ ions when they have been depleted. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? buffers are the key. a buffer system is a solution that resists a change in ph when acids or. A buffer (or buffered) solution is one that resists a.
from kyvokedymolyb.modellervefiyatlar.com
the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffers are the key. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. buffers are the key. A buffer (or buffered) solution is one that resists a. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. a buffer system is a solution that resists a change in ph when acids or. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and donate h+ ions when they have been depleted. When there are too many h + ions, a buffer. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers?
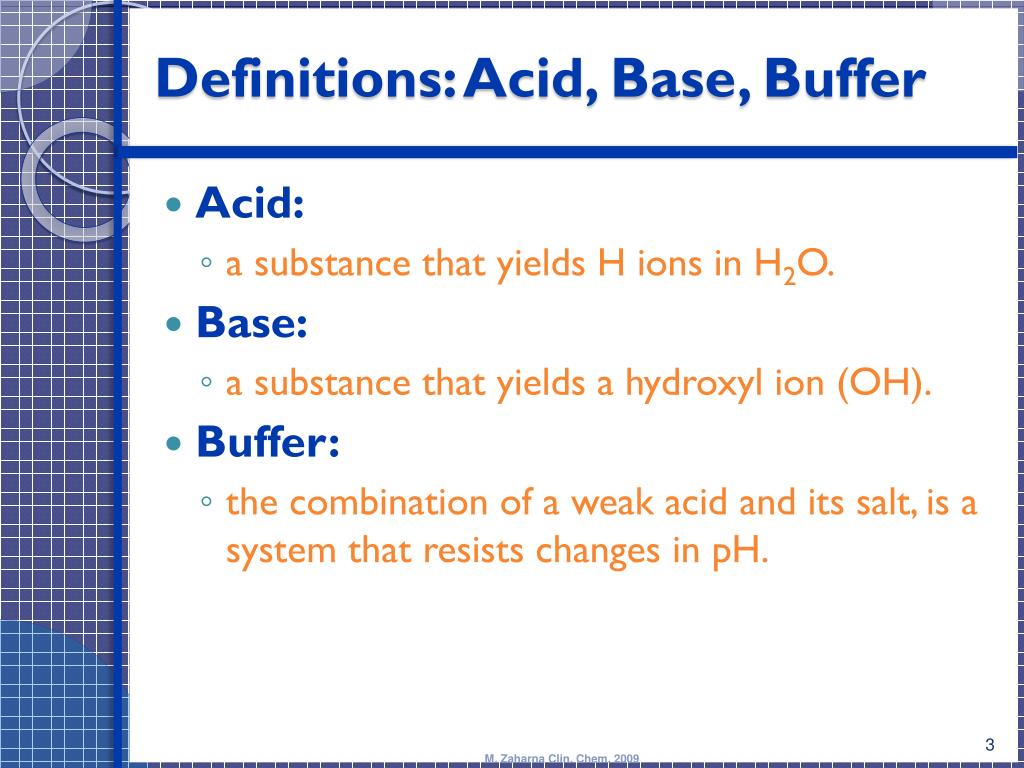
Acid Base Buffers
A Buffer Biology Quizlet buffers are the key. buffers are the key. a buffer system is a solution that resists a change in ph when acids or. buffers are the key. When there are too many h + ions, a buffer. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered) solution is one that resists a. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and donate h+ ions when they have been depleted.
From www.youtube.com
Buffers definition and the role of buffer in the body YouTube A Buffer Biology Quizlet the mechanism involves a buffer, a solution that resists dramatic changes in ph. When there are too many h + ions, a buffer. buffers are the key. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? buffers are the key. buffers, solutions that can resist changes in. A Buffer Biology Quizlet.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID A Buffer Biology Quizlet buffers are the key. buffers are the key. A buffer (or buffered) solution is one that resists a. a buffer system is a solution that resists a change in ph when acids or. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when. A Buffer Biology Quizlet.
From ar.inspiredpencil.com
Buffer Definition A Buffer Biology Quizlet the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. When there are too many h. A Buffer Biology Quizlet.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your A Buffer Biology Quizlet the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. buffers are the key. the mechanism involves a buffer, a solution that resists dramatic changes in ph. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? Insure that the body. A Buffer Biology Quizlet.
From api-stg.3m.com
💌 Define buffer biology. Buffers What are the Importance of Buffers in A Buffer Biology Quizlet When there are too many h + ions, a buffer. A buffer (or buffered) solution is one that resists a. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. Insure. A Buffer Biology Quizlet.
From www.chegg.com
Solved Consider the following questions about buffer A Buffer Biology Quizlet the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. buffers are the key. a buffer system is a solution that resists a change in ph when acids or. Insure that the body. A Buffer Biology Quizlet.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID A Buffer Biology Quizlet the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer system is a solution that resists a change in ph when acids or. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and donate. A Buffer Biology Quizlet.
From www.numerade.com
SOLVED Q The buffer systems that are the most important in the A Buffer Biology Quizlet buffers are the key. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? When there are too many h + ions, a buffer. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in. A Buffer Biology Quizlet.
From www.expii.com
Buffers — Definition & Overview Expii A Buffer Biology Quizlet a buffer system is a solution that resists a change in ph when acids or. buffers are the key. the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffers are the key. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? Insure that. A Buffer Biology Quizlet.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet A Buffer Biology Quizlet the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the mechanism involves a buffer, a solution that resists dramatic changes in ph. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in. A Buffer Biology Quizlet.
From quizlet.com
What is a buffer solution, and what are its properties? Quizlet A Buffer Biology Quizlet When there are too many h + ions, a buffer. buffers are the key. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when. A Buffer Biology Quizlet.
From www.aatbio.com
Buffers and Lab Consumables AAT Bioquest A Buffer Biology Quizlet buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. buffers are the key. When there are too many h + ions, a buffer. buffers are the key. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? . A Buffer Biology Quizlet.
From kyvokedymolyb.modellervefiyatlar.com
Acid Base Buffers A Buffer Biology Quizlet Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and donate h+ ions when they have been depleted. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the mechanism involves. A Buffer Biology Quizlet.
From www.youtube.com
Role of buffers in biological system YouTube A Buffer Biology Quizlet buffers are the key. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and donate h+ ions when they have been depleted. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers?. A Buffer Biology Quizlet.
From www.slideshare.net
Buffer system A Buffer Biology Quizlet When there are too many h + ions, a buffer. a buffer system is a solution that resists a change in ph when acids or. the mechanism involves a buffer, a solution that resists dramatic changes in ph. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph,. A Buffer Biology Quizlet.
From www.pinterest.com
What are the Buffers and its Importance? This article explains the A Buffer Biology Quizlet buffers are the key. A buffer (or buffered) solution is one that resists a. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? When there are too many h + ions, a buffer. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden. A Buffer Biology Quizlet.
From quizlet.com
biology biological molecules Diagram Quizlet A Buffer Biology Quizlet buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. When there are too many h + ions, a buffer. Insure that the body fluids do not fluctuate very much. A Buffer Biology Quizlet.
From www.youtube.com
Buffer in biological system buffer system in blood buffer in eyes A Buffer Biology Quizlet A buffer (or buffered) solution is one that resists a. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and donate h+ ions. A Buffer Biology Quizlet.
From www.onlinebiologynotes.com
Buffer, buffering capacity, properties of good buffer and role of A Buffer Biology Quizlet a buffer system is a solution that resists a change in ph when acids or. buffers are the key. When there are too many h + ions, a buffer. buffers are the key. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when. A Buffer Biology Quizlet.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance A Buffer Biology Quizlet buffers are the key. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and donate h+ ions when they have been depleted.. A Buffer Biology Quizlet.
From animalia-life.club
Phosphate Buffer System Equation A Buffer Biology Quizlet the mechanism involves a buffer, a solution that resists dramatic changes in ph. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. When there are too many h. A Buffer Biology Quizlet.
From www.pinterest.com
Buffer Buffer solution, Easy science, Flashcards A Buffer Biology Quizlet buffers are the key. A buffer (or buffered) solution is one that resists a. buffers are the key. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and donate h+ ions when they have been depleted. a buffer. A Buffer Biology Quizlet.
From quizlet.com
buffering system Diagram Quizlet A Buffer Biology Quizlet buffers are the key. buffers are the key. a buffer system is a solution that resists a change in ph when acids or. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. the most relevant systems for biology are the carbonic acid/carbonate buffering system,. A Buffer Biology Quizlet.
From studylib.net
Buffer Solutions A Buffer Biology Quizlet A buffer (or buffered) solution is one that resists a. the mechanism involves a buffer, a solution that resists dramatic changes in ph. When there are too many h + ions, a buffer. buffers are the key. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. study. A Buffer Biology Quizlet.
From www.slideshare.net
Buffers in chemical analysis, types of buffers A Buffer Biology Quizlet When there are too many h + ions, a buffer. a buffer system is a solution that resists a change in ph when acids or. buffers are the key. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and. A Buffer Biology Quizlet.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high A Buffer Biology Quizlet a buffer system is a solution that resists a change in ph when acids or. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. buffers are the key. buffers are the key. the mechanism involves a buffer, a solution that resists dramatic changes in. A Buffer Biology Quizlet.
From westudyhere.com
Example of a Buffer in Biology Biological Significance and Its A Buffer Biology Quizlet the mechanism involves a buffer, a solution that resists dramatic changes in ph. Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept h+ ions when they are in excess and donate h+ ions when they have been depleted. a buffer system is a solution that resists. A Buffer Biology Quizlet.
From bid.meetbirmingham.com
O Sistema Detectou A Saturação De Um Buffer EDULEARN A Buffer Biology Quizlet study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? buffers are the key. buffers are the key. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. A buffer (or buffered) solution is one that resists a. Insure that the. A Buffer Biology Quizlet.
From www.youtube.com
WCLN Buffers Biology YouTube A Buffer Biology Quizlet buffers are the key. a buffer system is a solution that resists a change in ph when acids or. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? Insure that the body fluids do not fluctuate very much in acid or base, prevent large sudden changes in ph, accept. A Buffer Biology Quizlet.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its A Buffer Biology Quizlet a buffer system is a solution that resists a change in ph when acids or. When there are too many h + ions, a buffer. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. buffers, solutions that can resist changes in ph, are key to maintaining stable h. A Buffer Biology Quizlet.
From www.youtube.com
Biological Buffers in Nature YouTube A Buffer Biology Quizlet When there are too many h + ions, a buffer. a buffer system is a solution that resists a change in ph when acids or. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. A buffer (or buffered) solution is one that resists a. buffers are. A Buffer Biology Quizlet.
From animalia-life.club
Phosphate Buffer System Equation A Buffer Biology Quizlet the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. When there are too many h + ions, a buffer. buffers, solutions that can resist changes in ph, are key to maintaining stable h + ion concentrations in biological systems. study with quizlet and memorize flashcards containing terms like. A Buffer Biology Quizlet.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination for Biologists A Buffer Biology Quizlet the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffers are the key. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? buffers are the key. A buffer (or buffered) solution is one that resists a. When there are too many h + ions,. A Buffer Biology Quizlet.
From www.youtube.com
Buffers and pH Biology YouTube A Buffer Biology Quizlet the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered) solution is one that resists a. buffers are the key. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? the most relevant systems for biology are the carbonic acid/carbonate buffering system,. A Buffer Biology Quizlet.
From www.brainkart.com
Buffers A Buffer Biology Quizlet the mechanism involves a buffer, a solution that resists dramatic changes in ph. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. study with quizlet and memorize flashcards containing terms like what do buffered solutions do?, what are buffers? buffers are the key. a buffer system. A Buffer Biology Quizlet.