Standard Enthalpy Of Formation Cl2 . the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. Elements in their standard state are not. Cl2 (g) → 2 cl (g) → 2. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. the standard enthalpy of formation for an element in its standard state is zero!!!!
from studylib.net
the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. the standard enthalpy of formation for an element in its standard state is zero!!!! Cl2 (g) → 2 cl (g) → 2. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. Elements in their standard state are not.
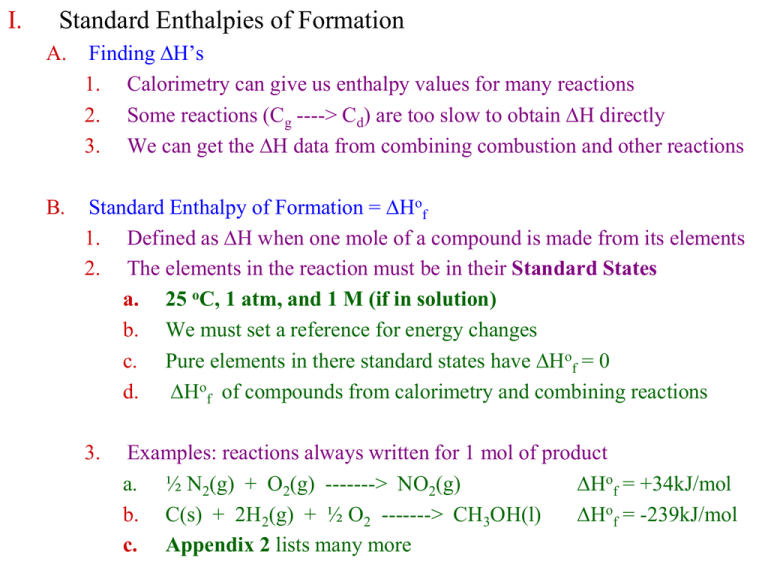
I. Standard Enthalpies of Formation
Standard Enthalpy Of Formation Cl2 enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. Elements in their standard state are not. Cl2 (g) → 2 cl (g) → 2. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. the standard enthalpy of formation for an element in its standard state is zero!!!! definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol.
From www.beinyu.com
Standard Enthalpy Of Formation Of Ethanol Standard Enthalpy Of Formation Cl2 Cl2 (g) → 2 cl (g) → 2. Elements in their standard state are not. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. the standard enthalpy of formation for an element in its. Standard Enthalpy Of Formation Cl2.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Cl2 Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. the standard enthalpy of formation for an element in its standard state is zero!!!! Cl2 (g) → 2 cl (g) → 2. Elements in their standard state are not. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. Standard Enthalpy Of Formation Cl2.
From www.numerade.com
SOLVED A scientist measures the standard enthalpy change for the Standard Enthalpy Of Formation Cl2 Elements in their standard state are not. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. Cl2 (g) → 2 cl (g) → 2. enthalpy of formation ( δhf) is the enthalpy change for the. Standard Enthalpy Of Formation Cl2.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Cl2 Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. the standard enthalpy of. Standard Enthalpy Of Formation Cl2.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Cl2 Cl2 (g) → 2 cl (g) → 2. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. Elements in their standard state are not. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. the standard enthalpy of formation. Standard Enthalpy Of Formation Cl2.
From learningcampusdirk.z13.web.core.windows.net
Standard Enthalpy Of Formation Chart Standard Enthalpy Of Formation Cl2 Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. the standard enthalpy of formation for an element in its standard state is zero!!!! the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. if we have values for the appropriate standard enthalpies of formation, we. Standard Enthalpy Of Formation Cl2.
From www.numerade.com
SOLVEDThe species which by definition has ZERO standard molar enthalpy Standard Enthalpy Of Formation Cl2 Cl2 (g) → 2 cl (g) → 2. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. the standard enthalpy of formation for an element in its standard state is zero!!!! Elements in their standard state are not. Δhr(0 k) =. Standard Enthalpy Of Formation Cl2.
From www.numerade.com
SOLVEDUse standard enthalpies of formation from Table 7.2 to determine Standard Enthalpy Of Formation Cl2 Elements in their standard state are not. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. Δhr(0. Standard Enthalpy Of Formation Cl2.
From www.bartleby.com
Answered Homework 3 • Use standard enthalpies of… bartleby Standard Enthalpy Of Formation Cl2 Elements in their standard state are not. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. the standard enthalpy of formation for an element in its standard state is zero!!!! enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a. Standard Enthalpy Of Formation Cl2.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Standard Enthalpy Of Formation Cl2 Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation for an element in its standard. Standard Enthalpy Of Formation Cl2.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Cl2 Cl2 (g) → 2 cl (g) → 2. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy. Standard Enthalpy Of Formation Cl2.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of Cl(aq) given Standard Enthalpy Of Formation Cl2 Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. Elements in their standard state are not. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance. Standard Enthalpy Of Formation Cl2.
From byjus.com
Bond dissociaton enthalpy of H2, Cl2 and HCl are 434, 242 and 431 kJ Standard Enthalpy Of Formation Cl2 if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. definition and explanation of the terms standard state and standard. Standard Enthalpy Of Formation Cl2.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 Standard Enthalpy Of Formation Cl2 definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Cl2 (g) → 2 cl (g) → 2. enthalpy of formation ( δhf) is the. Standard Enthalpy Of Formation Cl2.
From www.meritnation.com
Bond dissociation enthalpy of H2 , Cl2 and HCl are 434, 242 and 431 kJ Standard Enthalpy Of Formation Cl2 Cl2 (g) → 2 cl (g) → 2. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. Elements in their standard state are not. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound. Standard Enthalpy Of Formation Cl2.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Cl2 the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. the standard enthalpy of formation for an element in its standard state is zero!!!! Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. Cl2 (g) → 2 cl (g) → 2. Δhr(0 k) = 239.2425 ±. Standard Enthalpy Of Formation Cl2.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Cl2 the standard enthalpy of formation for an element in its standard state is zero!!!! if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values. Standard Enthalpy Of Formation Cl2.
From www.youtube.com
🔺️H for the reaction, F2 + 2HCl 》2HF +Cl2 is 352.8kj and standard Standard Enthalpy Of Formation Cl2 Elements in their standard state are not. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. enthalpy of formation ( δhf) is the enthalpy. Standard Enthalpy Of Formation Cl2.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of Cl(aq), given Standard Enthalpy Of Formation Cl2 if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1. Standard Enthalpy Of Formation Cl2.
From freddiewhittaker.z13.web.core.windows.net
Enthalpy Of Formation Chart Standard Enthalpy Of Formation Cl2 Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Δhr(298.15 k) = 242.6046 ± 0.0020. Standard Enthalpy Of Formation Cl2.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Cl2 the standard enthalpy of formation for an element in its standard state is zero!!!! the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any.. Standard Enthalpy Of Formation Cl2.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Cl2 if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. the standard enthalpy of formation for an element in its standard state is zero!!!! Elements in their standard state are not. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. enthalpy of formation ( δhf) is the enthalpy change for. Standard Enthalpy Of Formation Cl2.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Cl2 the standard enthalpy of formation for an element in its standard state is zero!!!! if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. Elements in their standard state are not. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a. Standard Enthalpy Of Formation Cl2.
From www.numerade.com
SOLVEDCalculate the standard enthalpy of formation of PCl5( s) from Standard Enthalpy Of Formation Cl2 Cl2 (g) → 2 cl (g) → 2. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound. Standard Enthalpy Of Formation Cl2.
From gmbar.co
️Heat Of Formation Worksheet Free Download Gmbar.co Standard Enthalpy Of Formation Cl2 Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. Elements in their standard state are not. the standard enthalpy of formation for an element in its standard state is zero!!!! Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. if. Standard Enthalpy Of Formation Cl2.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Cl2 the standard enthalpy of formation for an element in its standard state is zero!!!! Elements in their standard state are not. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. Cl2 (g) → 2 cl (g) → 2. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol. Standard Enthalpy Of Formation Cl2.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Cl2 Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. the standard enthalpy of formation for an element in its standard state is zero!!!! enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation Cl2.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Cl2 the standard enthalpy of formation for an element in its standard state is zero!!!! definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. . Standard Enthalpy Of Formation Cl2.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Cl2 if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. Cl2 (g) → 2 cl (g) → 2. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. definition and explanation of the terms standard state and. Standard Enthalpy Of Formation Cl2.
From www.chegg.com
Solved Calculate the enthalpy of the following reaction Standard Enthalpy Of Formation Cl2 definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the. Standard Enthalpy Of Formation Cl2.
From www.youtube.com
Standard Enthalpy of Formation Example 2 YouTube Standard Enthalpy Of Formation Cl2 Cl2 (g) → 2 cl (g) → 2. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation is defined as the change in enthalpy. Standard Enthalpy Of Formation Cl2.
From www.numerade.com
SOLVED Which is the correct equation corresponding to the standard Standard Enthalpy Of Formation Cl2 definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. the standard enthalpy of formation is defined as the change in enthalpy when. Standard Enthalpy Of Formation Cl2.
From www.chegg.com
Solved Calculate the standard enthalpy of formation of Standard Enthalpy Of Formation Cl2 Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. Elements in their standard state are not. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. Standard Enthalpy Of Formation Cl2.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Cl2 Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. Δhr(298.15 k) = 242.6046 ± 0.0020 kj/mol. Cl2 (g) → 2 cl (g) → 2. if we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a. Standard Enthalpy Of Formation Cl2.
From www.numerade.com
SOLVED Explain why the standard enthalpy of formation () for Cl2 (g Standard Enthalpy Of Formation Cl2 enthalpy of formation ( δhf) is the enthalpy change for the formation of 1 mol of a compound from its component. the standard enthalpy of formation for an element in its standard state is zero!!!! Δhr(0 k) = 239.2425 ± 0.0020 kj/mol. definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation Cl2.