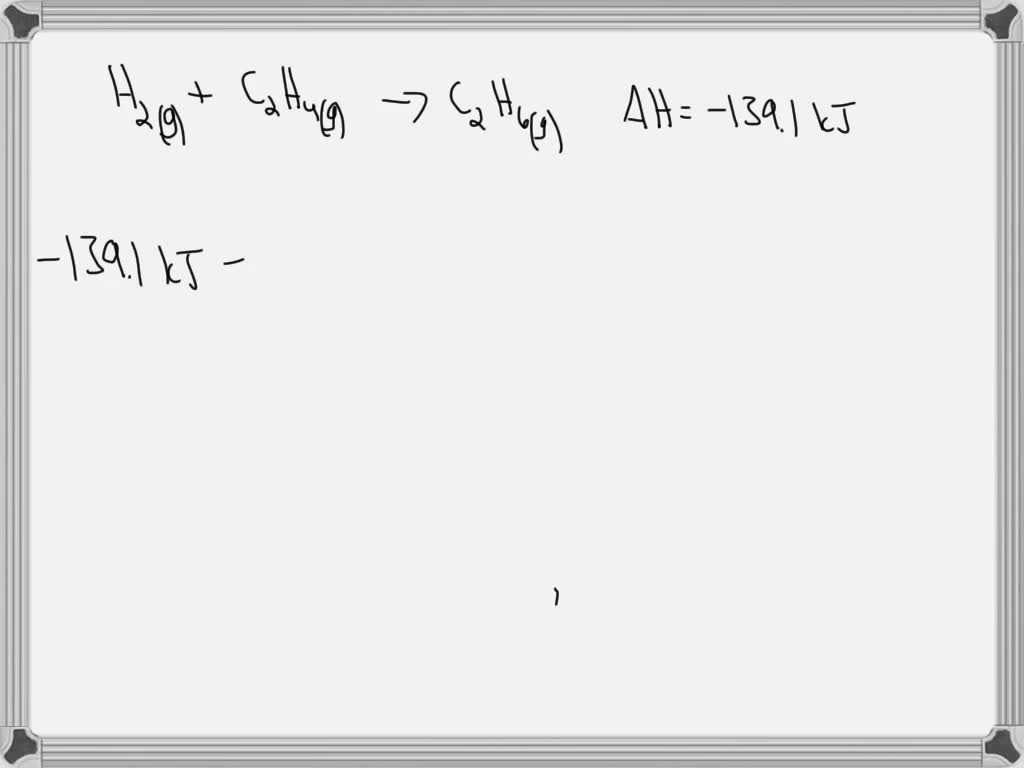Standard Enthalpy Of Formation Of Br2(G) . Br2 (cr,l) → br2 (g) →. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation of any element in its standard state is zero by definition. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. The enthalpy of formation for br (monoatomic gas) is. The enthalpy of formation for br 2 in its standard state is zero. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. consequently, br 2 (g) has a nonzero standard enthalpy of formation. That the element phosphorus is a unique case. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Δhr(0 k) = 45.68 ± 0.11 kj/mol.
from www.numerade.com
Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. The enthalpy of formation for br 2 in its standard state is zero. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Δhr(0 k) = 45.68 ± 0.11 kj/mol. Br2 (cr,l) → br2 (g) →. The enthalpy of formation for br (monoatomic gas) is. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. That the element phosphorus is a unique case. consequently, br 2 (g) has a nonzero standard enthalpy of formation.
SOLVED A scientist measures the standard enthalpy change for the
Standard Enthalpy Of Formation Of Br2(G) the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. consequently, br 2 (g) has a nonzero standard enthalpy of formation. the standard enthalpy of formation of any element in its standard state is zero by definition. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. That the element phosphorus is a unique case. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Δhr(0 k) = 45.68 ± 0.11 kj/mol. Br2 (cr,l) → br2 (g) →. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. The enthalpy of formation for br 2 in its standard state is zero. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. The enthalpy of formation for br (monoatomic gas) is.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib Standard Enthalpy Of Formation Of Br2(G) The enthalpy of formation for br 2 in its standard state is zero. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. The enthalpy of formation for. Standard Enthalpy Of Formation Of Br2(G).
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib Standard Enthalpy Of Formation Of Br2(G) Br2 (cr,l) → br2 (g) →. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. consequently, br 2 (g) has a nonzero standard enthalpy of formation. The enthalpy of formation for br 2 in its standard state is zero. Δhr(0 k) = 45.68 ± 0.11 kj/mol. the standard. Standard Enthalpy Of Formation Of Br2(G).
From questions.kunduz.com
1622 QUESTION 12 · 1 POINT Given the foll... Physical Chemistry Standard Enthalpy Of Formation Of Br2(G) The enthalpy of formation for br 2 in its standard state is zero. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. That the element phosphorus is a unique case. The enthalpy of formation for br (monoatomic gas) is. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. the standard enthalpy of formation of. Standard Enthalpy Of Formation Of Br2(G).
From nasirghopbuck.blogspot.com
How to Calculate the Standard Enthalpy of Combustion Standard Enthalpy Of Formation Of Br2(G) definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. The enthalpy of formation for br 2 in its standard state is zero. consequently, br 2 (g) has a nonzero standard enthalpy of formation. That the element phosphorus is a unique case. the standard enthalpy of formation of any. Standard Enthalpy Of Formation Of Br2(G).
From www.numerade.com
SOLVED The standard enthalpy of formation for the reaction below is Standard Enthalpy Of Formation Of Br2(G) consequently, br 2 (g) has a nonzero standard enthalpy of formation. the standard enthalpy of formation of any element in its standard state is zero by definition. The enthalpy of formation for br 2 in its standard state is zero. That the element phosphorus is a unique case. the standard enthalpy of formation is the enthalpy change. Standard Enthalpy Of Formation Of Br2(G).
From www.beinyu.com
Standard Enthalpy Of Formation Of Ethanol Standard Enthalpy Of Formation Of Br2(G) Δhr(0 k) = 45.68 ± 0.11 kj/mol. consequently, br 2 (g) has a nonzero standard enthalpy of formation. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. The enthalpy of formation for br (monoatomic gas) is. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure. Standard Enthalpy Of Formation Of Br2(G).
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Of Br2(G) the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. consequently, br 2 (g) has a nonzero standard enthalpy of formation. definition and explanation of the terms standard state. Standard Enthalpy Of Formation Of Br2(G).
From www.numerade.com
SOLVED The enthalpy change for the following reaction is 81.7 kJ I2(g Standard Enthalpy Of Formation Of Br2(G) definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. That the element phosphorus is a unique case. Br2 (cr,l) → br2 (g) →. The enthalpy of formation for br 2 in its standard state is zero. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. The enthalpy of formation for br (monoatomic. Standard Enthalpy Of Formation Of Br2(G).
From www.numerade.com
SOLVED Bromine gas and methanoic acid react according to the following Standard Enthalpy Of Formation Of Br2(G) Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. Δhr(0 k) = 45.68 ± 0.11 kj/mol. The enthalpy of formation for br 2 in. Standard Enthalpy Of Formation Of Br2(G).
From www.youtube.com
Temperature Br2(l)→Br2(g) Spontaneous YouTube Standard Enthalpy Of Formation Of Br2(G) Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. Δhr(0 k) = 45.68 ± 0.11 kj/mol. That the element phosphorus is a unique case. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements.. Standard Enthalpy Of Formation Of Br2(G).
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Of Br2(G) consequently, br 2 (g) has a nonzero standard enthalpy of formation. the standard enthalpy of formation of any element in its standard state is zero by definition. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. The enthalpy of formation for br 2 in its standard state is zero. definition. Standard Enthalpy Of Formation Of Br2(G).
From freddiewhittaker.z13.web.core.windows.net
Enthalpy Of Formation Chart Standard Enthalpy Of Formation Of Br2(G) The enthalpy of formation for br 2 in its standard state is zero. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. the standard enthalpy of formation of any element in its standard state is zero by definition. consequently, br 2 (g) has a. Standard Enthalpy Of Formation Of Br2(G).
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Of Br2(G) definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Br2 (cr,l) → br2 (g) →. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed. Standard Enthalpy Of Formation Of Br2(G).
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Of Br2(G) definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Br2 (cr,l) → br2 (g) →. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986. Standard Enthalpy Of Formation Of Br2(G).
From askfilo.com
Consider the following. (a) Standard enthalpy of formation of Br2 (I) is Standard Enthalpy Of Formation Of Br2(G) the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. Br2 (cr,l) → br2 (g) →. consequently, br 2 (g) has a nonzero standard enthalpy of formation. That the element phosphorus. Standard Enthalpy Of Formation Of Br2(G).
From www.chegg.com
Solved A scientist measures the standard enthalpy change for Standard Enthalpy Of Formation Of Br2(G) definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. consequently, br 2 (g) has a nonzero standard enthalpy of formation. The enthalpy of formation for br (monoatomic gas) is. That the element phosphorus is a unique case. the standard enthalpy of formation is the enthalpy change when 1. Standard Enthalpy Of Formation Of Br2(G).
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Of Br2(G) the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. That the element phosphorus is a unique case. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. consequently, br 2 (g) has a. Standard Enthalpy Of Formation Of Br2(G).
From nasirghopbuck.blogspot.com
How to Calculate the Standard Enthalpy of Combustion Standard Enthalpy Of Formation Of Br2(G) The enthalpy of formation for br (monoatomic gas) is. Br2 (cr,l) → br2 (g) →. The enthalpy of formation for br 2 in its standard state is zero. consequently, br 2 (g) has a nonzero standard enthalpy of formation. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. That the element phosphorus. Standard Enthalpy Of Formation Of Br2(G).
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Of Br2(G) That the element phosphorus is a unique case. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. Δhr(0 k) = 45.68 ± 0.11 kj/mol. consequently, br 2 (g) has a nonzero standard enthalpy of formation. Br2 (cr,l) → br2 (g) →. The enthalpy of formation for br (monoatomic gas) is. the standard enthalpy of formation of any element in its. Standard Enthalpy Of Formation Of Br2(G).
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Of Br2(G) consequently, br 2 (g) has a nonzero standard enthalpy of formation. Br2 (cr,l) → br2 (g) →. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. The enthalpy of formation for br 2 in its standard state is zero. Δhr(0 k) = 45.68 ± 0.11 kj/mol. That the element phosphorus is a. Standard Enthalpy Of Formation Of Br2(G).
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Of Br2(G) Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. The enthalpy of formation for br 2 in its standard state is zero. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. Δhr(0 k) = 45.68 ± 0.11 kj/mol. consequently, br 2 (g) has a nonzero standard enthalpy of formation. definition and explanation of. Standard Enthalpy Of Formation Of Br2(G).
From www.numerade.com
SOLVED (a) Calculate the standard enthalpy of formation of gaseous Standard Enthalpy Of Formation Of Br2(G) definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. The enthalpy of formation for br (monoatomic gas) is. Δhr(0 k) = 45.68 ± 0.11 kj/mol. The enthalpy of formation for br 2 in its standard state is zero. the standard enthalpy of formation is the enthalpy change when 1. Standard Enthalpy Of Formation Of Br2(G).
From www.youtube.com
Standard Enthalpy of Formation Example 2 YouTube Standard Enthalpy Of Formation Of Br2(G) The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. consequently, br 2 (g) has a nonzero standard enthalpy of formation. Δhr(0 k) = 45.68 ± 0.11 kj/mol. the standard enthalpy of formation of any element in its standard state is zero by definition. The enthalpy of formation for br (monoatomic gas). Standard Enthalpy Of Formation Of Br2(G).
From www.uz-gnesin-academy.ru
Standard enthalpy of formation table Standard Enthalpy Of Formation Of Br2(G) That the element phosphorus is a unique case. The enthalpy of formation for br 2 in its standard state is zero. the standard enthalpy of formation of any element in its standard state is zero by definition. Δhr(0 k) = 45.68 ± 0.11 kj/mol. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the. Standard Enthalpy Of Formation Of Br2(G).
From www.numerade.com
SOLVED A scientist measures the standard enthalpy change for the Standard Enthalpy Of Formation Of Br2(G) consequently, br 2 (g) has a nonzero standard enthalpy of formation. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. The enthalpy of formation for br. Standard Enthalpy Of Formation Of Br2(G).
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Of Br2(G) the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The enthalpy of formation for br (monoatomic gas) is. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard. Standard Enthalpy Of Formation Of Br2(G).
From www.chegg.com
Solved Table 1. Standard enthalpies of formation for Standard Enthalpy Of Formation Of Br2(G) consequently, br 2 (g) has a nonzero standard enthalpy of formation. the standard enthalpy of formation of any element in its standard state is zero by definition. Δhr(0 k) = 45.68 ± 0.11 kj/mol. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Δhr(298.15 k). Standard Enthalpy Of Formation Of Br2(G).
From ar.inspiredpencil.com
Enthalpy Of Reaction Table Standard Enthalpy Of Formation Of Br2(G) The enthalpy of formation for br 2 in its standard state is zero. That the element phosphorus is a unique case. consequently, br 2 (g) has a nonzero standard enthalpy of formation. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is the enthalpy change. Standard Enthalpy Of Formation Of Br2(G).
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Of Br2(G) Δhr(0 k) = 45.68 ± 0.11 kj/mol. consequently, br 2 (g) has a nonzero standard enthalpy of formation. The enthalpy of formation for br (monoatomic gas) is. Br2 (cr,l) → br2 (g) →. The enthalpy of formation for br 2 in its standard state is zero. That the element phosphorus is a unique case. Δhr(298.15 k) = 30.89 ±. Standard Enthalpy Of Formation Of Br2(G).
From mavink.com
Standard Enthalpy Of Combustion Chart Standard Enthalpy Of Formation Of Br2(G) definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Δhr(0 k) = 45.68 ± 0.11 kj/mol. The enthalpy of formation for br 2 in its standard state is zero. Br2 (cr,l) → br2 (g) →. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. the standard enthalpy of formation is the. Standard Enthalpy Of Formation Of Br2(G).
From www.numerade.com
SOLVEDThe species which by definition has ZERO standard molar enthalpy Standard Enthalpy Of Formation Of Br2(G) Br2 (cr,l) → br2 (g) →. The enthalpy of formation for br (monoatomic gas) is. Δhr(0 k) = 45.68 ± 0.11 kj/mol. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. consequently, br 2 (g) has a nonzero standard enthalpy of formation. The enthalpy of formation for br 2. Standard Enthalpy Of Formation Of Br2(G).
From brandonkss.github.io
Enthalpy Of Formation Chart Standard Enthalpy Of Formation Of Br2(G) The enthalpy of formation for br 2 in its standard state is zero. The enthalpy of formation for br (monoatomic gas) is. consequently, br 2 (g) has a nonzero standard enthalpy of formation. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. Br2 (cr,l) → br2 (g) →. the standard enthalpy of formation of any element in its standard state. Standard Enthalpy Of Formation Of Br2(G).
From www.toppr.com
Calculate the entropy of Br2(g) in the reaction H2(g) + Br2(g) 2HBr(g Standard Enthalpy Of Formation Of Br2(G) definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Δhr(0 k) = 45.68 ± 0.11 kj/mol. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. The enthalpy of formation for br (monoatomic gas) is. consequently, br 2 (g) has a nonzero standard enthalpy of formation. The enthalpy of formation for br. Standard Enthalpy Of Formation Of Br2(G).
From www.numerade.com
SOLVED Question No 5 Timer 0119 Mark 2 If the standard enthalpies Standard Enthalpy Of Formation Of Br2(G) The enthalpy of formation for br (monoatomic gas) is. consequently, br 2 (g) has a nonzero standard enthalpy of formation. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The enthalpy of formation for br 2 in its standard state is zero. Br2 (cr,l) → br2. Standard Enthalpy Of Formation Of Br2(G).
From www.chegg.com
Solved Calculate the enthalpy of the following reaction Standard Enthalpy Of Formation Of Br2(G) The reaction enthalpy was calculated nolan, lópez de la vega, et al., 1986 from the experimental. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. consequently, br 2 (g) has a nonzero standard enthalpy of formation. Δhr(298.15 k) = 30.89 ± 0.11 kj/mol. the standard. Standard Enthalpy Of Formation Of Br2(G).