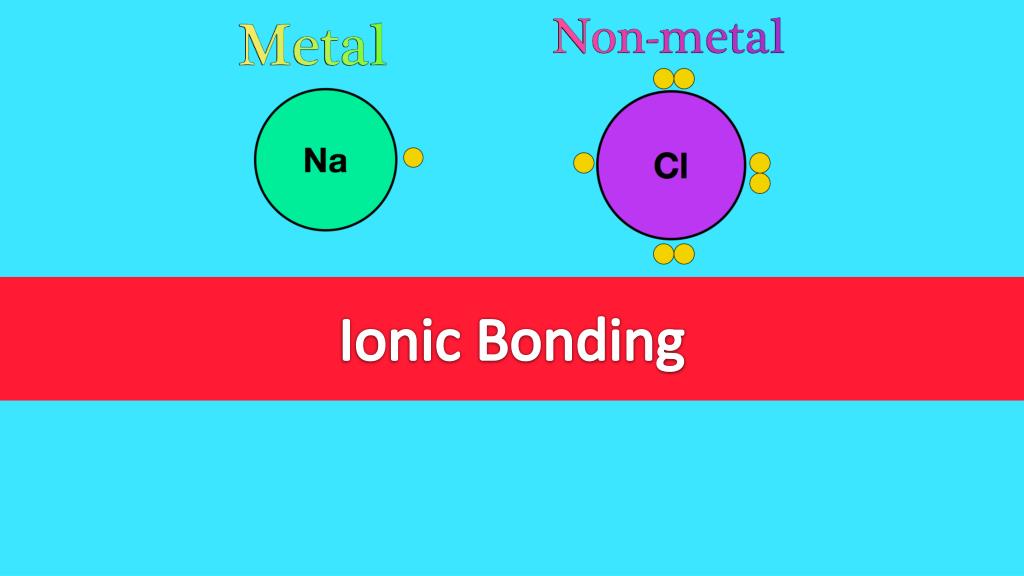
Describe Ionic Bonding . See examples of ionic bond formation and slideshow of ionic compounds. Learn more about ionic bonds, their. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. These oppositely charged ions attract each other to form ionic networks (or lattices). See examples of ionic compounds and how to predict ionic bonding using electronegativity values. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. We summarize the important points about ionic bonding: Learn what an ionic bond is, how it forms, and what properties it has.
from www.slideserve.com
An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. These oppositely charged ions attract each other to form ionic networks (or lattices). See examples of ionic bond formation and slideshow of ionic compounds. Learn more about ionic bonds, their. See examples of ionic compounds and how to predict ionic bonding using electronegativity values. Learn what an ionic bond is, how it forms, and what properties it has. We summarize the important points about ionic bonding:
PPT Ionic Bonding PowerPoint Presentation, free download ID2664362
Describe Ionic Bonding Learn more about ionic bonds, their. See examples of ionic compounds and how to predict ionic bonding using electronegativity values. Learn what an ionic bond is, how it forms, and what properties it has. An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. These oppositely charged ions attract each other to form ionic networks (or lattices). See examples of ionic bond formation and slideshow of ionic compounds. Learn more about ionic bonds, their. We summarize the important points about ionic bonding:
From www.dreamstime.com
Ionic Bond Vector Illustration. Labeled Diagram with Formation Describe Ionic Bonding We summarize the important points about ionic bonding: At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. See examples of ionic bond formation and slideshow of ionic compounds. See examples of ionic compounds and how to predict ionic bonding using electronegativity values. An ionic bond is a. Describe Ionic Bonding.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Describe Ionic Bonding Learn more about ionic bonds, their. See examples of ionic bond formation and slideshow of ionic compounds. An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. Learn what an ionic bond is, how it forms, and what properties it has. Compounds composed of ions are. Describe Ionic Bonding.
From www.slideserve.com
PPT Chapter 7 Ionic Bonding PowerPoint Presentation, free download Describe Ionic Bonding Learn what an ionic bond is, how it forms, and what properties it has. See examples of ionic bond formation and slideshow of ionic compounds. These oppositely charged ions attract each other to form ionic networks (or lattices). We summarize the important points about ionic bonding: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions. Describe Ionic Bonding.
From tuitiontube.com
Ionic Bond and Ionic Bond Formation, Definition, Properties in Describe Ionic Bonding See examples of ionic compounds and how to predict ionic bonding using electronegativity values. Learn what an ionic bond is, how it forms, and what properties it has. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Learn more about ionic bonds, their. See examples of ionic bond formation. Describe Ionic Bonding.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Describe Ionic Bonding We summarize the important points about ionic bonding: An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. See examples of ionic bond formation and slideshow of ionic compounds. Learn more about ionic bonds, their. Compounds composed of ions are called ionic compounds (or salts), and. Describe Ionic Bonding.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Describe Ionic Bonding At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. We summarize the important points about ionic bonding: Learn more about ionic bonds, their.. Describe Ionic Bonding.
From chemistry.about.com
Examples of Ionic Bonds and Ionic Compounds Describe Ionic Bonding An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions. Describe Ionic Bonding.
From edu.rsc.org
How to teach ionic bonding at 1416 CPD RSC Education Describe Ionic Bonding See examples of ionic bond formation and slideshow of ionic compounds. These oppositely charged ions attract each other to form ionic networks (or lattices). We summarize the important points about ionic bonding: At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. An ionic bond is a type. Describe Ionic Bonding.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Describe Ionic Bonding See examples of ionic compounds and how to predict ionic bonding using electronegativity values. Learn more about ionic bonds, their. These oppositely charged ions attract each other to form ionic networks (or lattices). At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Learn what an ionic bond. Describe Ionic Bonding.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Describe Ionic Bonding We summarize the important points about ionic bonding: See examples of ionic compounds and how to predict ionic bonding using electronegativity values. See examples of ionic bond formation and slideshow of ionic compounds. These oppositely charged ions attract each other to form ionic networks (or lattices). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions. Describe Ionic Bonding.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID4493576 Describe Ionic Bonding Learn more about ionic bonds, their. Learn what an ionic bond is, how it forms, and what properties it has. These oppositely charged ions attract each other to form ionic networks (or lattices). See examples of ionic compounds and how to predict ionic bonding using electronegativity values. An ionic bond is a type of chemical bond formed by the transfer. Describe Ionic Bonding.
From circuitdatadolorously.z14.web.core.windows.net
Lewis Diagrams For Ionic Compounds Describe Ionic Bonding Learn more about ionic bonds, their. We summarize the important points about ionic bonding: These oppositely charged ions attract each other to form ionic networks (or lattices). An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. Learn what an ionic bond is, how it forms,. Describe Ionic Bonding.
From www.slideserve.com
PPT Chapter 7 Ionic Bonding PowerPoint Presentation, free download Describe Ionic Bonding Learn more about ionic bonds, their. Learn what an ionic bond is, how it forms, and what properties it has. An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. See examples of ionic compounds and how to predict ionic bonding using electronegativity values. These oppositely. Describe Ionic Bonding.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID5602517 Describe Ionic Bonding These oppositely charged ions attract each other to form ionic networks (or lattices). At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. See examples of ionic compounds and how to predict ionic bonding using electronegativity values. Compounds composed of ions are called ionic compounds (or salts), and. Describe Ionic Bonding.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Describe Ionic Bonding These oppositely charged ions attract each other to form ionic networks (or lattices). We summarize the important points about ionic bonding: Learn what an ionic bond is, how it forms, and what properties it has. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Learn more about. Describe Ionic Bonding.
From www.slideserve.com
PPT Ionic bonding writing formulas PowerPoint Presentation, free Describe Ionic Bonding Learn more about ionic bonds, their. These oppositely charged ions attract each other to form ionic networks (or lattices). See examples of ionic compounds and how to predict ionic bonding using electronegativity values. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: See examples of ionic bond formation and. Describe Ionic Bonding.
From edu.rsc.org
How to teach ionic bonding at 1416 CPD RSC Education Describe Ionic Bonding See examples of ionic bond formation and slideshow of ionic compounds. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: See examples of ionic compounds and how to predict ionic bonding using electronegativity values. Learn more about ionic bonds, their. These oppositely charged ions attract each other to form. Describe Ionic Bonding.
From schematicmanualwilliam.z13.web.core.windows.net
Ionic Bonding Diagram Describe Ionic Bonding See examples of ionic compounds and how to predict ionic bonding using electronegativity values. Learn what an ionic bond is, how it forms, and what properties it has. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Learn more about ionic bonds, their. We summarize the important. Describe Ionic Bonding.
From myans.bhantedhammika.net
How To Describe Ionic Bonding Describe Ionic Bonding An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. Learn more about ionic bonds, their. See examples of ionic bond formation and slideshow of ionic compounds. At r 0 , the ions are more stable (have a lower potential energy) than they are at an. Describe Ionic Bonding.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Describe Ionic Bonding An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. See examples of ionic bond formation and slideshow of ionic compounds. See examples of ionic compounds and how to predict ionic bonding using electronegativity values. Compounds composed of ions are called ionic compounds (or salts), and. Describe Ionic Bonding.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2664362 Describe Ionic Bonding Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: See examples of ionic bond formation and slideshow of ionic compounds. We summarize the important points about ionic bonding: Learn what an ionic bond is, how it forms, and what properties it has. These oppositely charged ions attract each other. Describe Ionic Bonding.
From www.breakingatom.com
Ionic Bonding Describe Ionic Bonding See examples of ionic bond formation and slideshow of ionic compounds. We summarize the important points about ionic bonding: See examples of ionic compounds and how to predict ionic bonding using electronegativity values. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. These oppositely charged ions attract. Describe Ionic Bonding.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Describe Ionic Bonding See examples of ionic compounds and how to predict ionic bonding using electronegativity values. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. We summarize the important points about ionic bonding: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held. Describe Ionic Bonding.
From mrbarnestc.com
3. Ionic Bonding Mr Barnes Teaches Chemistry Describe Ionic Bonding Learn what an ionic bond is, how it forms, and what properties it has. Learn more about ionic bonds, their. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting. Describe Ionic Bonding.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Describe Ionic Bonding At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. See examples of ionic compounds and how to predict ionic bonding using electronegativity values. We summarize the important points about ionic bonding: These oppositely charged ions attract each other to form ionic networks (or lattices). Learn more about. Describe Ionic Bonding.
From en.wikipedia.org
Ionic bonding Wikipedia Describe Ionic Bonding Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: See examples of ionic bond formation and slideshow of ionic compounds. Learn what an ionic bond is, how it forms, and what properties it has. Learn more about ionic bonds, their. See examples of ionic compounds and how to predict. Describe Ionic Bonding.
From studylib.net
IONIC_BONDING Describe Ionic Bonding Learn what an ionic bond is, how it forms, and what properties it has. See examples of ionic bond formation and slideshow of ionic compounds. These oppositely charged ions attract each other to form ionic networks (or lattices). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: See examples. Describe Ionic Bonding.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Describe Ionic Bonding An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. Learn what an ionic bond is, how it forms, and what properties it has. These oppositely charged ions attract each other to form ionic networks (or lattices). See examples of ionic bond formation and slideshow of. Describe Ionic Bonding.
From www.slideserve.com
PPT CHEMICAL BONDING PART 1 IONIC BONDING PowerPoint Presentation Describe Ionic Bonding Learn what an ionic bond is, how it forms, and what properties it has. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions.. Describe Ionic Bonding.
From logan-has-small.blogspot.com
What is an Ionic Bond LoganhasSmall Describe Ionic Bonding These oppositely charged ions attract each other to form ionic networks (or lattices). An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. See examples of ionic compounds and how to predict ionic bonding using electronegativity values. See examples of ionic bond formation and slideshow of. Describe Ionic Bonding.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Describe Ionic Bonding See examples of ionic compounds and how to predict ionic bonding using electronegativity values. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. These oppositely charged ions attract each other to form ionic networks (or lattices). Learn what an ionic bond is, how it forms, and what. Describe Ionic Bonding.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Describe Ionic Bonding See examples of ionic bond formation and slideshow of ionic compounds. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. Learn what an ionic bond. Describe Ionic Bonding.
From dokumen.tips
(PPT) Ionic Bonding LO Describe ionic bonding in terms of Describe Ionic Bonding See examples of ionic bond formation and slideshow of ionic compounds. Learn more about ionic bonds, their. Learn what an ionic bond is, how it forms, and what properties it has. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. See examples of ionic compounds and how. Describe Ionic Bonding.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Describe Ionic Bonding Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. These oppositely charged ions attract each other to form ionic networks (or lattices). Learn more about ionic bonds,. Describe Ionic Bonding.
From sciencenotes.org
Ionic Bond Definition and Examples Describe Ionic Bonding An ionic bond is a type of chemical bond formed by the transfer of electrons between atoms, resulting in positively and negatively charged ions. These oppositely charged ions attract each other to form ionic networks (or lattices). We summarize the important points about ionic bonding: See examples of ionic compounds and how to predict ionic bonding using electronegativity values. Compounds. Describe Ionic Bonding.