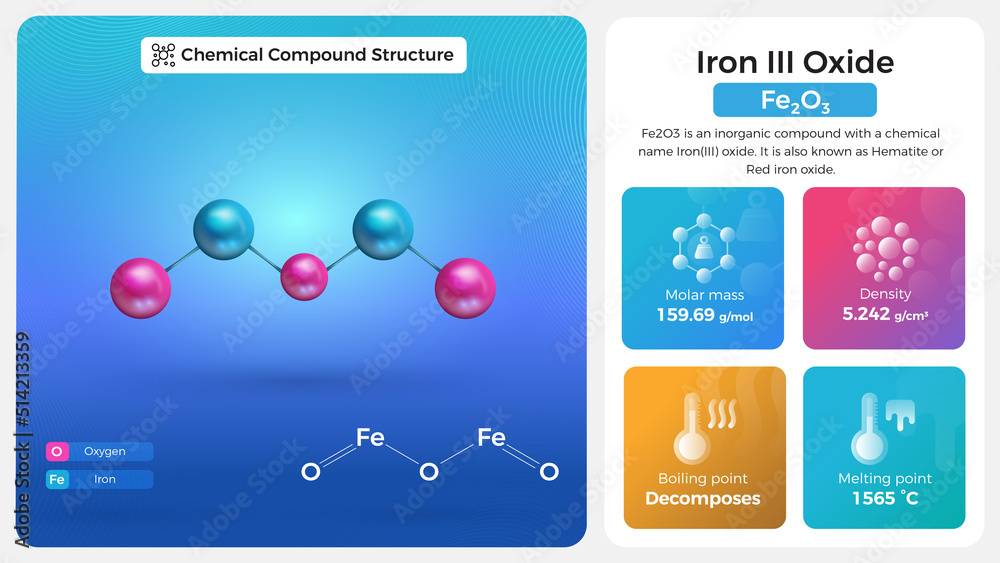
Iron Iii Oxide Solubility . Iron (iii) oxide is insoluble. Iron (iii) oxalate is, in practical situations, insoluble. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. It is noticeably ferromagnetic, accumulating thickly on the surface of. It is insoluble in water but readily reacts with acids. Since this fe has to be supplied via solution, the solubility. Iron (iii) oxide is a compound that appears in at least four different polymorphs: The ubiquitous nature of iron.
from stock.adobe.com
Since this fe has to be supplied via solution, the solubility. Iron (iii) oxide is a compound that appears in at least four different polymorphs: It is noticeably ferromagnetic, accumulating thickly on the surface of. The ubiquitous nature of iron. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Iron (iii) oxalate is, in practical situations, insoluble. It is insoluble in water but readily reacts with acids. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Iron (iii) oxide is insoluble.
iron III Oxide Properties and Chemical Compound Structure Stock Vector
Iron Iii Oxide Solubility In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Since this fe has to be supplied via solution, the solubility. Iron (iii) oxalate is, in practical situations, insoluble. The ubiquitous nature of iron. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Iron (iii) oxide is a compound that appears in at least four different polymorphs: It is noticeably ferromagnetic, accumulating thickly on the surface of. Iron (iii) oxide is insoluble. It is insoluble in water but readily reacts with acids.
From joigjogsq.blob.core.windows.net
Iron Iii Oxide Negative Ion at Emily Gaines blog Iron Iii Oxide Solubility The ubiquitous nature of iron. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Since this fe has to be supplied via solution, the solubility. Iron (iii) oxide is a compound that appears in at least four different polymorphs: It is. Iron Iii Oxide Solubility.
From www.researchgate.net
Mineral pigments in the iron(III) oxide/hydroxide system. Download Iron Iii Oxide Solubility The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. It is insoluble in water but readily reacts with acids. It is noticeably ferromagnetic, accumulating thickly. Iron Iii Oxide Solubility.
From www.researchgate.net
Influence of pH on the solubility of iron and ferric (hydro)oxide Iron Iii Oxide Solubility It is insoluble in water but readily reacts with acids. Since this fe has to be supplied via solution, the solubility. The ubiquitous nature of iron. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Iron (iii) oxide is a compound that appears in at least four different polymorphs: Iron (iii) oxalate is,. Iron Iii Oxide Solubility.
From stock.adobe.com
Iron Iii Oxide Properties and Chemical Compound Structure Stock Vector Iron Iii Oxide Solubility Iron (iii) oxide is insoluble. Iron (iii) oxide is a compound that appears in at least four different polymorphs: It is insoluble in water but readily reacts with acids. The ubiquitous nature of iron. Since this fe has to be supplied via solution, the solubility. Iron (iii) oxalate is, in practical situations, insoluble. It is noticeably ferromagnetic, accumulating thickly on. Iron Iii Oxide Solubility.
From www.slideserve.com
PPT Combustion analysis PowerPoint Presentation, free download ID Iron Iii Oxide Solubility In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. It is insoluble in water but readily reacts with acids. The ubiquitous nature of iron. Since this fe has to be supplied via solution, the solubility. Iron (iii) oxalate is, in practical situations, insoluble. The main source of iron for plants in most soils. Iron Iii Oxide Solubility.
From www.youtube.com
How to write the formula for iron (III) oxide YouTube Iron Iii Oxide Solubility The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. The ubiquitous nature of iron. It is noticeably ferromagnetic, accumulating thickly on the surface of. Since. Iron Iii Oxide Solubility.
From stock.adobe.com
iron III Oxide Properties and Chemical Compound Structure Stock Vector Iron Iii Oxide Solubility It is insoluble in water but readily reacts with acids. It is noticeably ferromagnetic, accumulating thickly on the surface of. Iron (iii) oxide is insoluble. Iron (iii) oxalate is, in practical situations, insoluble. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds. Iron Iii Oxide Solubility.
From www.researchgate.net
4. Solubility of iron species in equilibrium with iron oxides Iron Iii Oxide Solubility Iron (iii) oxide is a compound that appears in at least four different polymorphs: Iron (iii) oxide is insoluble. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. The ubiquitous nature of iron. Iron (iii) oxalate is, in practical situations, insoluble.. Iron Iii Oxide Solubility.
From www.researchgate.net
Solubility of ironoxyhydroxide phases. Equilibrium solubility of Iron Iii Oxide Solubility It is insoluble in water but readily reacts with acids. It is noticeably ferromagnetic, accumulating thickly on the surface of. The ubiquitous nature of iron. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Since this fe has to be supplied via solution, the solubility. Iron (iii) oxide is insoluble. The main source. Iron Iii Oxide Solubility.
From www.coursehero.com
[Solved] Iron (III) oxide is formed when iron combines with oxygen in Iron Iii Oxide Solubility It is noticeably ferromagnetic, accumulating thickly on the surface of. Since this fe has to be supplied via solution, the solubility. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. The ubiquitous nature of iron. Iron (iii) oxide is insoluble. Iron. Iron Iii Oxide Solubility.
From www.sciencephoto.com
Iron (III) oxide in water and in hydrochloric acid Stock Image C036 Iron Iii Oxide Solubility In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Iron (iii) oxalate is, in practical situations, insoluble. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. It is noticeably ferromagnetic, accumulating thickly on the. Iron Iii Oxide Solubility.
From www.showme.com
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe Iron Iii Oxide Solubility The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. The ubiquitous nature of iron. Iron (iii) oxalate is, in practical situations, insoluble. Iron (iii) oxide is insoluble. Since this fe has to be supplied via solution, the solubility. It is noticeably. Iron Iii Oxide Solubility.
From www.researchgate.net
Equilibrium solubility diagram of ferric hydrolysis species in the Iron Iii Oxide Solubility Iron (iii) oxide is a compound that appears in at least four different polymorphs: It is insoluble in water but readily reacts with acids. The ubiquitous nature of iron. Iron (iii) oxide is insoluble. It is noticeably ferromagnetic, accumulating thickly on the surface of. The main source of iron for plants in most soils are the iron(iii) oxides, here used. Iron Iii Oxide Solubility.
From mavink.com
Ph Solubility Curve Iron Iii Oxide Solubility Iron (iii) oxalate is, in practical situations, insoluble. Iron (iii) oxide is insoluble. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. It is insoluble in water but readily reacts with acids. In most soils, feiii oxides (group name) are the. Iron Iii Oxide Solubility.
From www.youtube.com
QUALITATIVE ANALYSIS 1.Test for iron III ion Fe³+. Three ways to Iron Iii Oxide Solubility Iron (iii) oxalate is, in practical situations, insoluble. It is noticeably ferromagnetic, accumulating thickly on the surface of. Since this fe has to be supplied via solution, the solubility. Iron (iii) oxide is insoluble. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), °. Iron Iii Oxide Solubility.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Iron Iii Oxide Solubility It is insoluble in water but readily reacts with acids. The ubiquitous nature of iron. It is noticeably ferromagnetic, accumulating thickly on the surface of. Iron (iii) oxide is insoluble. Since this fe has to be supplied via solution, the solubility. Iron (iii) oxalate is, in practical situations, insoluble. The main source of iron for plants in most soils are. Iron Iii Oxide Solubility.
From www.numerade.com
SOLVEDA sample consisting of a mixture of iron and iron(III) oxide was Iron Iii Oxide Solubility The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Iron (iii) oxide is insoluble. It is insoluble in water but readily reacts with acids. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Iron. Iron Iii Oxide Solubility.
From saylordotorg.github.io
Solubility and pH Iron Iii Oxide Solubility The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. The ubiquitous nature of iron. It is insoluble in water but readily reacts with acids. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Since. Iron Iii Oxide Solubility.
From www.numerade.com
SOLVED47. Iron(III) oxide reacts with carbon monoxide equation Iron Iii Oxide Solubility The ubiquitous nature of iron. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Since this fe has to be supplied via solution, the solubility.. Iron Iii Oxide Solubility.
From testbook.com
Iron(III) Oxide Formula Know Its Preparation, Properties & Uses Iron Iii Oxide Solubility Since this fe has to be supplied via solution, the solubility. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. The ubiquitous nature of iron.. Iron Iii Oxide Solubility.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses Iron Iii Oxide Solubility In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Iron (iii) oxalate is, in practical situations, insoluble. Since this fe has to be supplied via solution, the solubility. The ubiquitous nature of iron. Iron (iii) oxide is insoluble. It is noticeably ferromagnetic, accumulating thickly on the surface of. The main source of iron. Iron Iii Oxide Solubility.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Iron Iii Oxide Solubility The ubiquitous nature of iron. Iron (iii) oxide is insoluble. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Since this fe has to be supplied via solution, the solubility. It is noticeably ferromagnetic, accumulating thickly on the surface of. It. Iron Iii Oxide Solubility.
From www.researchgate.net
ETA curves of iron(III) oxide samples prepared by heating of various Iron Iii Oxide Solubility The ubiquitous nature of iron. It is noticeably ferromagnetic, accumulating thickly on the surface of. Iron (iii) oxide is insoluble. It is insoluble in water but readily reacts with acids. Iron (iii) oxide is a compound that appears in at least four different polymorphs: Since this fe has to be supplied via solution, the solubility. Iron (iii) oxalate is, in. Iron Iii Oxide Solubility.
From www.researchgate.net
EhpH diagram for iron, showing fields of solubility of dissolved Iron Iii Oxide Solubility Iron (iii) oxalate is, in practical situations, insoluble. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Since this fe has to be supplied via solution, the solubility. In most soils, feiii oxides (group name) are the common source of fe. Iron Iii Oxide Solubility.
From www.slideserve.com
PPT Naming Type (I) and (II) Compounds PowerPoint Presentation, free Iron Iii Oxide Solubility Since this fe has to be supplied via solution, the solubility. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. It is insoluble in water but readily reacts with acids. Iron (iii) oxide is a compound that appears in at least. Iron Iii Oxide Solubility.
From www.youtube.com
Formation of Rust Hydrated Iron (III) Oxide YouTube Iron Iii Oxide Solubility It is insoluble in water but readily reacts with acids. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Iron (iii) oxide is a compound that appears in at least four different polymorphs: It is noticeably ferromagnetic, accumulating thickly on the. Iron Iii Oxide Solubility.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to Iron Iii Oxide Solubility In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Iron (iii) oxide is insoluble. The ubiquitous nature of iron. Iron (iii) oxide is a compound that appears in at least four different polymorphs: Since this fe has to be supplied via solution, the solubility. The main source of iron for plants in most. Iron Iii Oxide Solubility.
From www.researchgate.net
Solubility of iron salts in HCl and H 2 SO 4 at 0°C Download Iron Iii Oxide Solubility The ubiquitous nature of iron. It is insoluble in water but readily reacts with acids. Iron (iii) oxide is a compound that appears in at least four different polymorphs: The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Iron (iii) oxide. Iron Iii Oxide Solubility.
From www.toppr.com
Iron III Oxide Formula Definition, Concepts and Examples Iron Iii Oxide Solubility Iron (iii) oxide is a compound that appears in at least four different polymorphs: Since this fe has to be supplied via solution, the solubility. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Iron (iii) oxalate is, in practical situations,. Iron Iii Oxide Solubility.
From www.researchgate.net
Influence of pH on the solubility of iron and ferric (hydro)oxide Iron Iii Oxide Solubility Iron (iii) oxide is a compound that appears in at least four different polymorphs: Iron (iii) oxalate is, in practical situations, insoluble. Iron (iii) oxide is insoluble. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. Since this fe has to be supplied via solution, the solubility. The main source of iron for. Iron Iii Oxide Solubility.
From www.sciencephoto.com
Iron (III) oxide in water Stock Image C036/3131 Science Photo Library Iron Iii Oxide Solubility Iron (iii) oxide is a compound that appears in at least four different polymorphs: Iron (iii) oxalate is, in practical situations, insoluble. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. In most soils, feiii oxides (group name) are the common. Iron Iii Oxide Solubility.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube Iron Iii Oxide Solubility Iron (iii) oxide is insoluble. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Iron (iii) oxide is a compound that appears in at least four different polymorphs: In most soils, feiii oxides (group name) are the common source of fe. Iron Iii Oxide Solubility.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library Iron Iii Oxide Solubility The ubiquitous nature of iron. Since this fe has to be supplied via solution, the solubility. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. It is insoluble in water but readily reacts with acids. Iron (iii) oxide is insoluble. Iron (iii) oxalate is, in practical situations, insoluble. The main source of iron. Iron Iii Oxide Solubility.
From www.numerade.com
SOLVEDThe formation of rust [Iron (III) oxide] on the surface of iron Iron Iii Oxide Solubility Iron (iii) oxalate is, in practical situations, insoluble. In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. The main source of iron for plants in most soils are the iron(iii) oxides, here used as a group name for all tially of fe(iii), ° compounds consisting. Iron (iii) oxide is a compound that appears. Iron Iii Oxide Solubility.
From www.youtube.com
What is the formula for iron III oxide? YouTube Iron Iii Oxide Solubility In most soils, feiii oxides (group name) are the common source of fe for plant nutrition. It is insoluble in water but readily reacts with acids. The ubiquitous nature of iron. Iron (iii) oxide is a compound that appears in at least four different polymorphs: Iron (iii) oxalate is, in practical situations, insoluble. Iron (iii) oxide is insoluble. It is. Iron Iii Oxide Solubility.