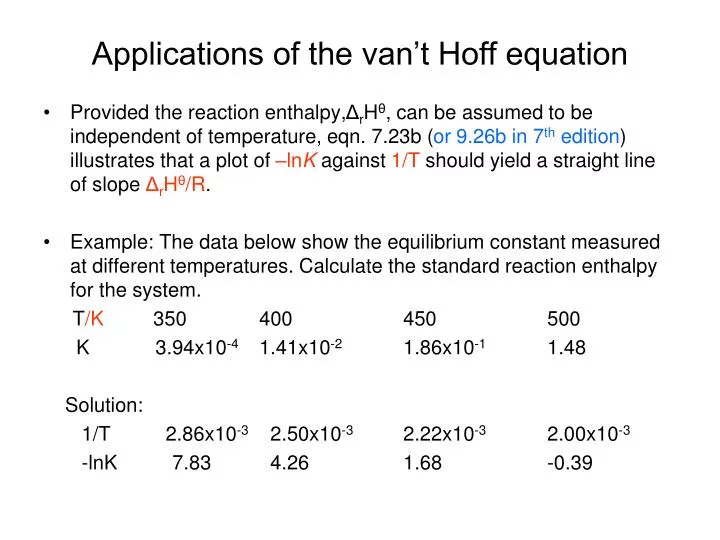
Van't Hoff Factor Of Magnesium Chloride . Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of. Α = 0.80 n = 2. Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units 2) solve for the osmotic. The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions. 1) calculate the van 't hoff factor from the degree of dissociation: 2.7 (versus an ideal value of 3 Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. I = 1 + 0.80 (2 − 1) i = 1.80. The van 't hoff factor of.
from www.slideserve.com
Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of. Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). The van 't hoff factor of. 2) solve for the osmotic. I = 1 + 0.80 (2 − 1) i = 1.80. 2.7 (versus an ideal value of 3 Α = 0.80 n = 2. The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions.
PPT Applications of the van’t Hoff equation PowerPoint Presentation
Van't Hoff Factor Of Magnesium Chloride I = 1 + 0.80 (2 − 1) i = 1.80. Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). The van 't hoff factor of. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. I = 1 + 0.80 (2 − 1) i = 1.80. It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units 2) solve for the osmotic. 1) calculate the van 't hoff factor from the degree of dissociation: 2.7 (versus an ideal value of 3 Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of. The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions. Α = 0.80 n = 2.
From id.hutomosungkar.com
13+ How To Find Van T Hoff Factor Today Hutomo Van't Hoff Factor Of Magnesium Chloride 2) solve for the osmotic. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions. It refers to the ratio of moles of particles formed when a substance. Van't Hoff Factor Of Magnesium Chloride.
From www.youtube.com
Ecuación de VAN'T HOFF ejercicio resuelto (ejemplo 1) YouTube Van't Hoff Factor Of Magnesium Chloride Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). 2) solve for the osmotic. 2.7 (versus an ideal value of 3 1) calculate the van 't hoff factor from the degree of dissociation: It refers to the ratio of moles of particles formed when a substance dissolves in a solution. Van't Hoff Factor Of Magnesium Chloride.
From chemistnotes.com
Osmosis/Reverse osmosis/osmotic pressure/Definition/Equation Van't Hoff Factor Of Magnesium Chloride Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). The van 't hoff factor of. 1) calculate the van 't hoff factor from the degree of dissociation: Α = 0.80 n = 2. I = 1 + 0.80 (2 − 1) i = 1.80. Magnesium chloride in water calculate the. Van't Hoff Factor Of Magnesium Chloride.
From www.elgencurioso.com
Factor de van't Hoff Definición y cómo calcularlo El Gen Curioso Van't Hoff Factor Of Magnesium Chloride I = 1 + 0.80 (2 − 1) i = 1.80. 2.7 (versus an ideal value of 3 Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions. 1) calculate the van 't hoff factor. Van't Hoff Factor Of Magnesium Chloride.
From www.slideserve.com
PPT Properties of Solutions PowerPoint Presentation, free download Van't Hoff Factor Of Magnesium Chloride It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units Α = 0.80 n = 2. 1) calculate the van 't hoff factor from the degree of dissociation: Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of. The van 't. Van't Hoff Factor Of Magnesium Chloride.
From www.slideserve.com
PPT Applications of the van’t Hoff equation PowerPoint Presentation Van't Hoff Factor Of Magnesium Chloride The van 't hoff factor of. Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). 2.7 (versus an ideal value of 3 Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. It refers. Van't Hoff Factor Of Magnesium Chloride.
From www.youtube.com
CHEM 201 Calculating Actual van't Hoff Factor YouTube Van't Hoff Factor Of Magnesium Chloride The van 't hoff factor of. The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions. 2.7 (versus an ideal value of 3 Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. 1) calculate the van 't. Van't Hoff Factor Of Magnesium Chloride.
From www.numerade.com
SOLVEDThe van't Hoff factor (i), is a measure of association or Van't Hoff Factor Of Magnesium Chloride Α = 0.80 n = 2. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions. The van 't hoff factor of. I = 1 + 0.80 (2. Van't Hoff Factor Of Magnesium Chloride.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry Van't Hoff Factor Of Magnesium Chloride The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. 2) solve for the osmotic. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m. Van't Hoff Factor Of Magnesium Chloride.
From byjus.com
what is the need and applicability of vant hoff factor Van't Hoff Factor Of Magnesium Chloride 2.7 (versus an ideal value of 3 Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units The van 't hoff. Van't Hoff Factor Of Magnesium Chloride.
From www.chegg.com
Solved I need help finding the van't hoff factor for MgCl2. Van't Hoff Factor Of Magnesium Chloride Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. 2.7 (versus an ideal value of 3 The van 't hoff factor of. It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of. Van't Hoff Factor Of Magnesium Chloride.
From www.youtube.com
van’t Hoff Equation Derivation Step by Step (easy) 💪 YouTube Van't Hoff Factor Of Magnesium Chloride 1) calculate the van 't hoff factor from the degree of dissociation: The van 't hoff factor of. It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has. Van't Hoff Factor Of Magnesium Chloride.
From quizretentives.z13.web.core.windows.net
How To Know The Vant Hoff Factor Van't Hoff Factor Of Magnesium Chloride I = 1 + 0.80 (2 − 1) i = 1.80. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of. 2.7 (versus an ideal value of 3 Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c.. Van't Hoff Factor Of Magnesium Chloride.
From www.toppr.com
Abnormal Masses and Van't Hoff Factor Definition, Examples, Diagrams Van't Hoff Factor Of Magnesium Chloride It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units Α = 0.80 n = 2. I = 1 + 0.80 (2 − 1) i = 1.80. 1) calculate the van 't hoff factor from the degree of dissociation: Magnesium chloride in water calculate the van’t hoff. Van't Hoff Factor Of Magnesium Chloride.
From www.youtube.com
Factor de van't Hoff 21062021 YouTube Van't Hoff Factor Of Magnesium Chloride Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. I = 1 + 0.80 (2 − 1) i = 1.80. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of. The van’t hoff factor provides an understanding. Van't Hoff Factor Of Magnesium Chloride.
From www.chegg.com
Solved Magnesium Chloride calorimeter experiment freezing Van't Hoff Factor Of Magnesium Chloride Α = 0.80 n = 2. Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). I = 1 + 0.80 (2 − 1) i = 1.80. The van 't hoff factor of. 2.7 (versus an ideal value of 3 The van’t hoff factor provides an understanding of how solutes impact. Van't Hoff Factor Of Magnesium Chloride.
From calculatorgwx.blogspot.com
Van T Hoff Equation Calculator CALCULLATOR GWX Van't Hoff Factor Of Magnesium Chloride The van 't hoff factor of. 2.7 (versus an ideal value of 3 2) solve for the osmotic. Α = 0.80 n = 2. It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units 1) calculate the van 't hoff factor from the degree of dissociation: Magnesium. Van't Hoff Factor Of Magnesium Chloride.
From www.slideserve.com
PPT Chapter 14 Solutions and Their Properties If you’re not part of Van't Hoff Factor Of Magnesium Chloride Α = 0.80 n = 2. 2.7 (versus an ideal value of 3 Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of. The van 't hoff factor of. 1) calculate the van 't hoff. Van't Hoff Factor Of Magnesium Chloride.
From www.numerade.com
SOLVED Using the van't Hoff factors in Table 12.7, calculate the mass Van't Hoff Factor Of Magnesium Chloride 1) calculate the van 't hoff factor from the degree of dissociation: It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units Α = 0.80 n = 2. I = 1 + 0.80 (2 − 1) i = 1.80. Magnesium chloride in water calculate the van’t hoff. Van't Hoff Factor Of Magnesium Chloride.
From www.chegg.com
Solved Exercise 14.87 Use the van't Hoff factors in the Van't Hoff Factor Of Magnesium Chloride Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that. Van't Hoff Factor Of Magnesium Chloride.
From www.numerade.com
SOLVED The van't Hoff factor for MgCl2 is 2.7. What is the boiling Van't Hoff Factor Of Magnesium Chloride Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units Calculate the boiling point of a 4.81% by mass solution of. Van't Hoff Factor Of Magnesium Chloride.
From www.chegg.com
Solved ab DataDetermine van't Hoff factor for magnesium Van't Hoff Factor Of Magnesium Chloride Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). I = 1 + 0.80 (2 − 1) i = 1.80. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. It refers to the. Van't Hoff Factor Of Magnesium Chloride.
From quizretentives.z13.web.core.windows.net
How To Know The Vant Hoff Factor Van't Hoff Factor Of Magnesium Chloride The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of. 1) calculate the van 't hoff factor from the degree of dissociation: 2.7 (versus an ideal value of 3 It refers to the ratio of moles of particles. Van't Hoff Factor Of Magnesium Chloride.
From howcarspecs.blogspot.com
What Is Vant Hoff Factor How Car Specs Van't Hoff Factor Of Magnesium Chloride 2) solve for the osmotic. Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). Α = 0.80 n = 2. The van 't hoff factor of. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of. I = 1 + 0.80 (2 − 1). Van't Hoff Factor Of Magnesium Chloride.
From www.slideserve.com
PPT COLLIGATIVE PROPERTIES PowerPoint Presentation, free download Van't Hoff Factor Of Magnesium Chloride 1) calculate the van 't hoff factor from the degree of dissociation: It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units 2.7 (versus an ideal value of 3 The van 't hoff factor of. The van’t hoff factor provides an understanding of how solutes impact the. Van't Hoff Factor Of Magnesium Chloride.
From www.slideserve.com
PPT Chapter 14 Solutions and Their Properties If you’re not part of Van't Hoff Factor Of Magnesium Chloride I = 1 + 0.80 (2 − 1) i = 1.80. Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). Α = 0.80 n = 2. The van 't hoff factor of. It refers to the ratio of moles of particles formed when a substance dissolves in a solution to. Van't Hoff Factor Of Magnesium Chloride.
From 876kendrawhitekabar.blogspot.com
Formula To Calculate Van't Hoff Factor Van't Hoff Factor Of Magnesium Chloride I = 1 + 0.80 (2 − 1) i = 1.80. It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c.. Van't Hoff Factor Of Magnesium Chloride.
From www.numerade.com
SOLVED Question 14 (6.66667 points) For which of the following has the Van't Hoff Factor Of Magnesium Chloride The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions. Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. It refers to the ratio of moles of particles formed when a substance dissolves in a solution to. Van't Hoff Factor Of Magnesium Chloride.
From quizretentives.z13.web.core.windows.net
What Is Van't Hoff Factor Class 12 Van't Hoff Factor Of Magnesium Chloride Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of. 2.7 (versus. Van't Hoff Factor Of Magnesium Chloride.
From www.numerade.com
SOLVED I need help in this question, please. What is the theoretical Van't Hoff Factor Of Magnesium Chloride I = 1 + 0.80 (2 − 1) i = 1.80. The van 't hoff factor of. 2.7 (versus an ideal value of 3 Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. Calculate the boiling point of a 4.81% by mass solution. Van't Hoff Factor Of Magnesium Chloride.
From www.youtube.com
Van't hoff factor and its significance YouTube Van't Hoff Factor Of Magnesium Chloride It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. Magnesium chloride in water calculate the van’t hoff factor for a. Van't Hoff Factor Of Magnesium Chloride.
From everipedia.org
Van 't Hoff equation Wiki Everipedia Van't Hoff Factor Of Magnesium Chloride I = 1 + 0.80 (2 − 1) i = 1.80. 2.7 (versus an ideal value of 3 1) calculate the van 't hoff factor from the degree of dissociation: Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). The van 't hoff factor of. Magnesium chloride in water calculate. Van't Hoff Factor Of Magnesium Chloride.
From www.numerade.com
SOLVEDFor 5 points, a 25.0 gram sample of Magnesium Chloride (MgClz Van't Hoff Factor Of Magnesium Chloride Magnesium chloride in water calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\) that has a measured freezing point of −0.25 c. Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). I = 1 + 0.80 (2 − 1) i = 1.80. 2) solve for the. Van't Hoff Factor Of Magnesium Chloride.
From slideplayer.com
Solutions Part ppt download Van't Hoff Factor Of Magnesium Chloride It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units 2) solve for the osmotic. Calculate the boiling point of a 4.81% by mass solution of magnesium chloride (mgcl 2, mw = 95.211 g/mol). Α = 0.80 n = 2. The van’t hoff factor provides an understanding. Van't Hoff Factor Of Magnesium Chloride.
From www.slideserve.com
PPT Chapter 13 Solutions part II Colligative Properties Van't Hoff Factor Of Magnesium Chloride The van 't hoff factor of. 1) calculate the van 't hoff factor from the degree of dissociation: It refers to the ratio of moles of particles formed when a substance dissolves in a solution to the moles of formula units The van’t hoff factor provides an understanding of how solutes impact the colligative properties of solutions. I = 1. Van't Hoff Factor Of Magnesium Chloride.