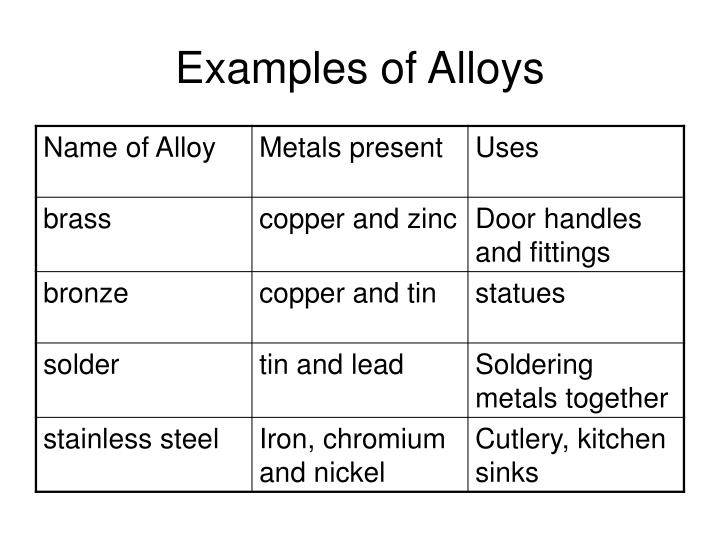
Alloy Examples In Chemistry . examples of alloys. mixtures of metals are called alloys, and they have tre. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. The components of alloys are. Alloys can be formed by. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. alloy, metallic substance composed of two or more elements, as either a compound or a solution. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon.
from www.slideserve.com
examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. Alloys can be formed by. mixtures of metals are called alloys, and they have tre. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. alloy, metallic substance composed of two or more elements, as either a compound or a solution. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. examples of alloys. The components of alloys are.
PPT Physical Properties of Metals PowerPoint Presentation ID5519685
Alloy Examples In Chemistry alloy, metallic substance composed of two or more elements, as either a compound or a solution. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. mixtures of metals are called alloys, and they have tre. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. Alloys can be formed by. The components of alloys are. alloy, metallic substance composed of two or more elements, as either a compound or a solution. examples of alloys. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to.
From www.examplesof.net
Example of Alloys Uses of Alloys in Daily Life Chemistry Lesson Plans Alloy Examples In Chemistry The components of alloys are. alloy, metallic substance composed of two or more elements, as either a compound or a solution. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. examples of alloys. examples. Alloy Examples In Chemistry.
From fyoskuafd.blob.core.windows.net
Alloy Examples Chemistry at David Hou blog Alloy Examples In Chemistry examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. alloy, metallic substance composed of two or more elements, as either a compound or a solution. The components of alloys are. Alloys can be formed by. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. examples of alloys include red. Alloy Examples In Chemistry.
From scienceinfo.com
Alloys Characteristics, Classification, Types, Benefits, Limitations Alloy Examples In Chemistry an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. examples of alloys. alloy, metallic substance composed of two or more elements, as either a compound or a solution. Alloys can be formed by. mixtures. Alloy Examples In Chemistry.
From www.youtube.com
Alloys & Their Properties Chemistry YouTube Alloy Examples In Chemistry 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. alloy, metallic substance composed of two or more elements, as either a compound or a solution. mixtures of metals are called alloys, and they have tre. The components of alloys are. Alloys can be formed. Alloy Examples In Chemistry.
From scienceneo.com
Alloys Definition, Properties, Formation, Facts, and RealWorld Alloy Examples In Chemistry examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. Alloys can be formed by. examples of alloys. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and. Alloy Examples In Chemistry.
From www.tes.com
Alloys Edexcel 91 Separate (Triple) Science Teaching Resources Alloy Examples In Chemistry an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. The components of alloys are. Alloys can be formed by. alloy, metallic substance composed of two or more elements, as either a compound or. Alloy Examples In Chemistry.
From www.researchgate.net
CHEMICAL COMPOSITION OF ALUMINIUM ALLOY 6063 Download Table Alloy Examples In Chemistry The components of alloys are. examples of alloys. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. alloy, metallic substance composed. Alloy Examples In Chemistry.
From scienceinfo.com
Alloys Characteristics, Classification, Types, Benefits, Limitations Alloy Examples In Chemistry Alloys can be formed by. alloy, metallic substance composed of two or more elements, as either a compound or a solution. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. examples of. Alloy Examples In Chemistry.
From fyoekanyx.blob.core.windows.net
Aluminum Tin Alloy at Michael Haman blog Alloy Examples In Chemistry The components of alloys are. examples of alloys. mixtures of metals are called alloys, and they have tre. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. alloy, metallic substance composed of two or more elements, as either a compound or a solution. examples of alloys include red gold (gold. Alloy Examples In Chemistry.
From www.pinterest.com
What Is an Alloy? Definition and Examples Chemistry, Alloy, Materials Alloy Examples In Chemistry alloy, metallic substance composed of two or more elements, as either a compound or a solution. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements.. Alloy Examples In Chemistry.
From www.expii.com
Alloys — Overview & Examples Expii Alloy Examples In Chemistry an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. mixtures of metals are called alloys, and they have tre. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. examples. Alloy Examples In Chemistry.
From www.slideserve.com
PPT Grade 9 Chemistry Unit PowerPoint Presentation, free download Alloy Examples In Chemistry The components of alloys are. alloy, metallic substance composed of two or more elements, as either a compound or a solution. mixtures of metals are called alloys, and they have tre. Alloys can be formed by. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. 25 rows generally, alloys are stronger and harder than. Alloy Examples In Chemistry.
From www.teachoo.com
Metals, Non Metals and Metalloids Meaning & Difference Teachoo Alloy Examples In Chemistry examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. Alloys can be formed by. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. mixtures of metals are called alloys, and they have tre. 25 rows generally, alloys are. Alloy Examples In Chemistry.
From hxelqrnxs.blob.core.windows.net
Mo Metal Name at William Hopkins blog Alloy Examples In Chemistry 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver. Alloy Examples In Chemistry.
From www.vrogue.co
What Is An Alloy Definition And Examples vrogue.co Alloy Examples In Chemistry mixtures of metals are called alloys, and they have tre. examples of alloys. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. alloy, metallic substance composed of two. Alloy Examples In Chemistry.
From www.vrogue.co
What Is An Alloy Definition And Examples vrogue.co Alloy Examples In Chemistry mixtures of metals are called alloys, and they have tre. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. The components of alloys are. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. Alloys can be formed by. examples of alloys.. Alloy Examples In Chemistry.
From www.slideshare.net
Alloy Alloy Examples In Chemistry 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. examples of alloys. alloy, metallic substance composed of two or more elements, as either a compound or a solution. The components of alloys are. mixtures of metals are called alloys, and they have tre.. Alloy Examples In Chemistry.
From animalia-life.club
Brass Alloy Composition Alloy Examples In Chemistry Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder. Alloy Examples In Chemistry.
From www.slideshare.net
Alloy Alloy Examples In Chemistry alloy, metallic substance composed of two or more elements, as either a compound or a solution. mixtures of metals are called alloys, and they have tre. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. examples of alloys. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. . Alloy Examples In Chemistry.
From www.animalia-life.club
Alloys Chemistry Alloy Examples In Chemistry 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. mixtures of metals are called alloys, and they have. Alloy Examples In Chemistry.
From www.techglads.com
Properties of alloys in Engineering Chemistry Tech Glads Alloy Examples In Chemistry an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Alloys can be formed by. Familiar examples of alloys include brass, bronze, stainless steel, 14k gold, sterling silver, and cast. mixtures of metals are called alloys, and they have tre. examples of alloys. alloy, metallic substance composed. Alloy Examples In Chemistry.
From www.thoughtco.com
Properties, Composition, and Production of Metal Alloys Alloy Examples In Chemistry an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. Familiar examples of alloys include. Alloy Examples In Chemistry.
From www.animalia-life.club
Alloys Chemistry Alloy Examples In Chemistry examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. Alloys can be formed by. mixtures of metals are called alloys, and they have tre. The components of alloys are. examples of alloys. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Familiar examples of. Alloy Examples In Chemistry.
From cikguwong.blogspot.com
EduMission Chemistry Form 4 Chapter 9 Making of Alloy Alloy Examples In Chemistry an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. examples of alloys. alloy, metallic substance composed of two or more elements, as either a. Alloy Examples In Chemistry.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C2.2 ac Metals and nonmetals Alloy Examples In Chemistry Alloys can be formed by. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. 25 rows generally, alloys are stronger and harder than their main. Alloy Examples In Chemistry.
From www.thoughtco.com
Metals Quiz Metal Properties and Chemistry Alloy Examples In Chemistry The components of alloys are. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. examples of alloys. mixtures of metals are called alloys, and they have tre. alloy, metallic substance composed of two or more elements, as either a compound or a solution. Alloys can be formed by. examples of alloys include red. Alloy Examples In Chemistry.
From gamesmartz.com
Alloy Definition Easy to Understand GameSmartz Alloy Examples In Chemistry examples of alloys. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. alloy, metallic substance composed of two or more elements, as either a compound or a solution. an alloy is. Alloy Examples In Chemistry.
From www.expii.com
Alloys — Overview & Examples Expii Alloy Examples In Chemistry examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. examples of alloys. . Alloy Examples In Chemistry.
From www.slideserve.com
PPT Alloys PowerPoint Presentation, free download ID2872003 Alloy Examples In Chemistry The components of alloys are. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc. mixtures of metals are called alloys, and they have tre. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. examples of alloys. Familiar examples of alloys include. Alloy Examples In Chemistry.
From www.thoughtco.com
Alloy Definition and Examples in Chemistry Alloy Examples In Chemistry an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. Alloys can be formed by. examples of alloys are brass, bronze, stainless steel, duralumin, nichrome, etc.. Alloy Examples In Chemistry.
From www.vrogue.co
What Is An Alloy Definition And Examples vrogue.co Alloy Examples In Chemistry examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. an alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to. Alloy Examples In Chemistry.
From ar.inspiredpencil.com
Alloy Chemistry Example Alloy Examples In Chemistry examples of alloys. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. alloy, metallic substance composed of two or more elements, as either a compound or a solution. an alloy is a mixture of metals that has bulk metallic properties different from those. Alloy Examples In Chemistry.
From ar.inspiredpencil.com
Alloy Chemistry Example Alloy Examples In Chemistry 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. alloy, metallic substance composed of two or more elements, as either a compound or a solution. mixtures of metals are called alloys, and they have tre. an alloy is a mixture of metals that. Alloy Examples In Chemistry.
From www.slideserve.com
PPT Physical Properties of Metals PowerPoint Presentation ID5519685 Alloy Examples In Chemistry examples of alloys. alloy, metallic substance composed of two or more elements, as either a compound or a solution. examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder. Alloy Examples In Chemistry.
From www.animalia-life.club
Alloys Chemistry Alloy Examples In Chemistry examples of alloys include red gold (gold and copper), white gold (gold and silver), sterling silver (silver and copper), steel or silicon. 25 rows generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to. The components of alloys are. an alloy is a mixture of metals that. Alloy Examples In Chemistry.