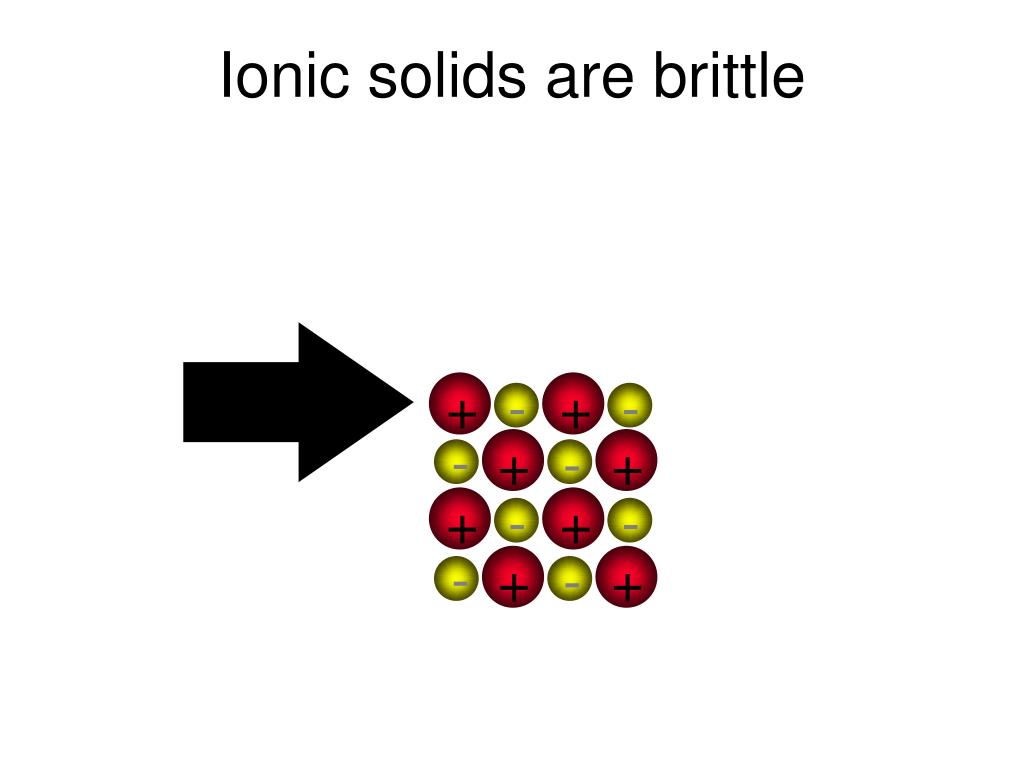
Brittle Ionic Compounds With Oxygen . Sodium chloride is taken as a typical ionic compound. ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic solids exhibit a crystalline structure and. How the ions are arranged in sodium chloride. What happens when an electric current. Oxygen is a group 16 element, and its atoms gain two. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Why are melting points high for ionic compounds? atoms tend to obey the octet rule when they react and form ionic compounds. the properties of ionic compounds shed some light on the nature of ionic bonds. diagram showing the consequence of shearing force applied on an ionic lattice structure, leading to like charged ions repelling each other, accounting for. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. why are ionic compounds brittle?
from www.slideserve.com
Ionic compounds dissociate into ions when dissolved in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Oxygen is a group 16 element, and its atoms gain two. ionic compounds are hard and brittle. How the ions are arranged in sodium chloride. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. the properties of ionic compounds shed some light on the nature of ionic bonds. atoms tend to obey the octet rule when they react and form ionic compounds. Sodium chloride is taken as a typical ionic compound. Why are melting points high for ionic compounds?
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint
Brittle Ionic Compounds With Oxygen diagram showing the consequence of shearing force applied on an ionic lattice structure, leading to like charged ions repelling each other, accounting for. Ionic compounds dissociate into ions when dissolved in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. What happens when an electric current. ionic compounds are hard and brittle. Why are melting points high for ionic compounds? compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. the properties of ionic compounds shed some light on the nature of ionic bonds. diagram showing the consequence of shearing force applied on an ionic lattice structure, leading to like charged ions repelling each other, accounting for. why are ionic compounds brittle? How the ions are arranged in sodium chloride. Ionic solids exhibit a crystalline structure and. Sodium chloride is taken as a typical ionic compound. Oxygen is a group 16 element, and its atoms gain two. atoms tend to obey the octet rule when they react and form ionic compounds.
From www.youtube.com
Why ionic solids are brittle? YouTube Brittle Ionic Compounds With Oxygen why are ionic compounds brittle? ionic compounds are hard and brittle. Oxygen is a group 16 element, and its atoms gain two. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. How the ions are arranged in sodium chloride. Sodium chloride is taken as a typical ionic. Brittle Ionic Compounds With Oxygen.
From www.numerade.com
SOLVED Why does the ceramic made from Thorium and Oxygen have the Brittle Ionic Compounds With Oxygen ionic compounds are hard and brittle. diagram showing the consequence of shearing force applied on an ionic lattice structure, leading to like charged ions repelling each other, accounting for. the properties of ionic compounds shed some light on the nature of ionic bonds. atoms tend to obey the octet rule when they react and form ionic. Brittle Ionic Compounds With Oxygen.
From exykbkxug.blob.core.windows.net
Brittle Ionic Compounds at Diana Glass blog Brittle Ionic Compounds With Oxygen compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. the properties of ionic compounds shed some light on the nature of ionic bonds. Sodium chloride is taken as a typical ionic compound. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. What. Brittle Ionic Compounds With Oxygen.
From www.numerade.com
SOLVED (c) K forms the compound KzO,which is an ionic compound that is Brittle Ionic Compounds With Oxygen ionic compounds are hard and brittle. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. What happens when an electric current. Ionic solids exhibit a crystalline structure and. How the ions are arranged in sodium chloride. why are ionic compounds brittle? Solutions of ionic compounds and melted. Brittle Ionic Compounds With Oxygen.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Brittle Ionic Compounds With Oxygen Oxygen is a group 16 element, and its atoms gain two. Ionic compounds dissociate into ions when dissolved in water. Sodium chloride is taken as a typical ionic compound. ionic compounds are hard and brittle. How the ions are arranged in sodium chloride. Ionic compounds have high melting and boiling points, so they are in the solid state at. Brittle Ionic Compounds With Oxygen.
From www.studocu.com
Covalent Compounds Worksheet Ionic compounds are brittle, covalent Brittle Ionic Compounds With Oxygen How the ions are arranged in sodium chloride. Oxygen is a group 16 element, and its atoms gain two. Sodium chloride is taken as a typical ionic compound. the properties of ionic compounds shed some light on the nature of ionic bonds. ionic compounds are hard and brittle. atoms tend to obey the octet rule when they. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Structure and Properties of Bonds ppt download Brittle Ionic Compounds With Oxygen compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Ionic compounds dissociate into ions when dissolved in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. atoms tend to obey the octet rule when they react and form ionic compounds. Oxygen. Brittle Ionic Compounds With Oxygen.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Brittle Ionic Compounds With Oxygen Oxygen is a group 16 element, and its atoms gain two. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. diagram showing the consequence of shearing force applied on an ionic lattice structure, leading to. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Ionic Compounds Ch.6 & ppt download Brittle Ionic Compounds With Oxygen Ionic solids exhibit a crystalline structure and. What happens when an electric current. atoms tend to obey the octet rule when they react and form ionic compounds. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Oxygen is a group 16 element, and its atoms gain two. diagram showing. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Ionic Bonding/Naming. ppt download Brittle Ionic Compounds With Oxygen Why are melting points high for ionic compounds? Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. atoms tend to obey the octet rule when they react and form ionic compounds. What happens when an electric current. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Matter & Bonding Lesson 2 ppt download Brittle Ionic Compounds With Oxygen Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic solids exhibit a crystalline structure and. Sodium chloride is taken as a typical ionic compound. why are ionic compounds brittle? Solutions of ionic compounds and melted ionic compounds conduct electricity, but. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Metallic Bonding. ppt download Brittle Ionic Compounds With Oxygen why are ionic compounds brittle? ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Sodium chloride is taken as a typical ionic compound. How the ions are arranged in sodium chloride. diagram showing the consequence of shearing force applied on an ionic lattice structure, leading to like charged ions repelling each. Brittle Ionic Compounds With Oxygen.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Brittle Ionic Compounds With Oxygen the properties of ionic compounds shed some light on the nature of ionic bonds. ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. What happens when an electric current. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Why are melting points high. Brittle Ionic Compounds With Oxygen.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Brittle Ionic Compounds With Oxygen compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Why are melting points high for ionic compounds? Oxygen is a group 16 element, and its atoms gain two. Ionic solids exhibit a crystalline structure and. atoms tend to obey the octet rule when they react and form ionic. Brittle Ionic Compounds With Oxygen.
From www.slideserve.com
PPT Properties of Substances PowerPoint Presentation, free download Brittle Ionic Compounds With Oxygen ionic compounds are hard and brittle. What happens when an electric current. Oxygen is a group 16 element, and its atoms gain two. why are ionic compounds brittle? compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Why are melting points high for ionic compounds? atoms. Brittle Ionic Compounds With Oxygen.
From hxeeheshc.blob.core.windows.net
Does Boron Not Form Ionic Compounds at Allen Birch blog Brittle Ionic Compounds With Oxygen How the ions are arranged in sodium chloride. Ionic solids exhibit a crystalline structure and. atoms tend to obey the octet rule when they react and form ionic compounds. the properties of ionic compounds shed some light on the nature of ionic bonds. What happens when an electric current. why are ionic compounds brittle? Ionic compounds dissociate. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Brittle Ionic Compounds With Oxygen Oxygen is a group 16 element, and its atoms gain two. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. atoms tend to obey the octet rule when they react and form ionic compounds. the properties of ionic compounds shed some light on the nature of ionic bonds. ionic compounds are. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Mr. Conkey Physical Science Ch ppt download Brittle Ionic Compounds With Oxygen Oxygen is a group 16 element, and its atoms gain two. why are ionic compounds brittle? Ionic solids exhibit a crystalline structure and. diagram showing the consequence of shearing force applied on an ionic lattice structure, leading to like charged ions repelling each other, accounting for. What happens when an electric current. atoms tend to obey the. Brittle Ionic Compounds With Oxygen.
From brainly.com
Which property is a characteristic of an ionic compound? low melting Brittle Ionic Compounds With Oxygen Sodium chloride is taken as a typical ionic compound. Ionic solids exhibit a crystalline structure and. why are ionic compounds brittle? Ionic compounds dissociate into ions when dissolved in water. What happens when an electric current. atoms tend to obey the octet rule when they react and form ionic compounds. How the ions are arranged in sodium chloride.. Brittle Ionic Compounds With Oxygen.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation ID1130121 Brittle Ionic Compounds With Oxygen ionic compounds are hard and brittle. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. How the ions are arranged in sodium chloride. Ionic solids exhibit a crystalline structure and. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Oxygen is a group 16. Brittle Ionic Compounds With Oxygen.
From www.slideserve.com
PPT Formation of Compounds PowerPoint Presentation, free download Brittle Ionic Compounds With Oxygen Oxygen is a group 16 element, and its atoms gain two. the properties of ionic compounds shed some light on the nature of ionic bonds. Why are melting points high for ionic compounds? How the ions are arranged in sodium chloride. atoms tend to obey the octet rule when they react and form ionic compounds. Ionic compounds dissociate. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Ionic Bonding. ppt download Brittle Ionic Compounds With Oxygen Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Oxygen is a group 16 element, and its atoms gain two. Why are melting points high for ionic. Brittle Ionic Compounds With Oxygen.
From quizdbthermoform.z13.web.core.windows.net
Why Are Ionic Compounds Stable Brittle Ionic Compounds With Oxygen Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. atoms tend to obey the octet rule when they react and form ionic compounds. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. compounds composed. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Chapter ppt download Brittle Ionic Compounds With Oxygen How the ions are arranged in sodium chloride. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. the properties of ionic compounds shed some light on the nature of ionic bonds. ionic compounds are hard and brittle. atoms tend to obey the octet rule when they react and form ionic compounds.. Brittle Ionic Compounds With Oxygen.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Brittle Ionic Compounds With Oxygen ionic compounds are hard and brittle. What happens when an electric current. Sodium chloride is taken as a typical ionic compound. diagram showing the consequence of shearing force applied on an ionic lattice structure, leading to like charged ions repelling each other, accounting for. Oxygen is a group 16 element, and its atoms gain two. why are. Brittle Ionic Compounds With Oxygen.
From www.researchgate.net
Hard and soft acids and bases, and ionic, van der Waals and covalent Brittle Ionic Compounds With Oxygen ionic compounds are hard and brittle. Ionic solids exhibit a crystalline structure and. why are ionic compounds brittle? How the ions are arranged in sodium chloride. Oxygen is a group 16 element, and its atoms gain two. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. What happens when. Brittle Ionic Compounds With Oxygen.
From brainly.in
Why ionic crystals are brittle in nature? Brainly.in Brittle Ionic Compounds With Oxygen diagram showing the consequence of shearing force applied on an ionic lattice structure, leading to like charged ions repelling each other, accounting for. Sodium chloride is taken as a typical ionic compound. Oxygen is a group 16 element, and its atoms gain two. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are. Brittle Ionic Compounds With Oxygen.
From www.slideserve.com
PPT 4.5 Physical Properties of Giant Ionic Compounds PowerPoint Brittle Ionic Compounds With Oxygen ionic compounds are hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. What happens when an electric current. Sodium chloride is taken as a typical ionic compound. Oxygen is a group 16 element, and its atoms gain two. atoms tend to obey the octet rule when they react and. Brittle Ionic Compounds With Oxygen.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5980343 Brittle Ionic Compounds With Oxygen the properties of ionic compounds shed some light on the nature of ionic bonds. why are ionic compounds brittle? Ionic solids exhibit a crystalline structure and. Oxygen is a group 16 element, and its atoms gain two. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. ionic compounds are hard and. Brittle Ionic Compounds With Oxygen.
From www.slideserve.com
PPT Ionic and Covalent bonds PowerPoint Presentation, free download Brittle Ionic Compounds With Oxygen Ionic compounds dissociate into ions when dissolved in water. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. How the ions are arranged in sodium chloride. why are ionic compounds brittle? Ionic compounds have high melting and boiling points, so they are in the solid state at room. Brittle Ionic Compounds With Oxygen.
From slidetodoc.com
TYPES OF CHEMICAL BONDS IONIC BONDS COVALENT BONDS Brittle Ionic Compounds With Oxygen Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. atoms tend to obey the octet rule when they react and form ionic compounds. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. ionic compounds are hard and brittle. What happens when an electric. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Ionic and Metallic Bonds ppt download Brittle Ionic Compounds With Oxygen What happens when an electric current. Sodium chloride is taken as a typical ionic compound. Ionic compounds dissociate into ions when dissolved in water. ionic compounds are hard and brittle. atoms tend to obey the octet rule when they react and form ionic compounds. why are ionic compounds brittle? Ionic compounds have high melting and boiling points,. Brittle Ionic Compounds With Oxygen.
From slideplayer.com
Ionic and Covalent Bonds ppt download Brittle Ionic Compounds With Oxygen the properties of ionic compounds shed some light on the nature of ionic bonds. Sodium chloride is taken as a typical ionic compound. ionic compounds are hard and brittle. atoms tend to obey the octet rule when they react and form ionic compounds. How the ions are arranged in sodium chloride. why are ionic compounds brittle?. Brittle Ionic Compounds With Oxygen.
From ar.inspiredpencil.com
Covalent Compound Examples Brittle Ionic Compounds With Oxygen compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Why are melting points high for ionic compounds? why are ionic compounds brittle? Sodium chloride is taken as a typical ionic compound. atoms tend to obey the octet rule when they react and form ionic compounds. the. Brittle Ionic Compounds With Oxygen.
From www.slideserve.com
PPT BC Science Connections 9 Unit 2 The electron arrangement of Brittle Ionic Compounds With Oxygen Why are melting points high for ionic compounds? Ionic solids exhibit a crystalline structure and. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. why are ionic compounds brittle? atoms tend to. Brittle Ionic Compounds With Oxygen.