Medical Device Clinical Trial Design . Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: Better trial design and improve the quality of data that may better. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. Guidance should help manufacturers select appropriate trial design. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. In the eu, all class iii. In this chapter tips are given on how to perform and manage global clinical trials. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. This review describes the fda.
from crfweb.com
This review describes the fda. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. Better trial design and improve the quality of data that may better. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. In the eu, all class iii. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: In this chapter tips are given on how to perform and manage global clinical trials. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. Guidance should help manufacturers select appropriate trial design.
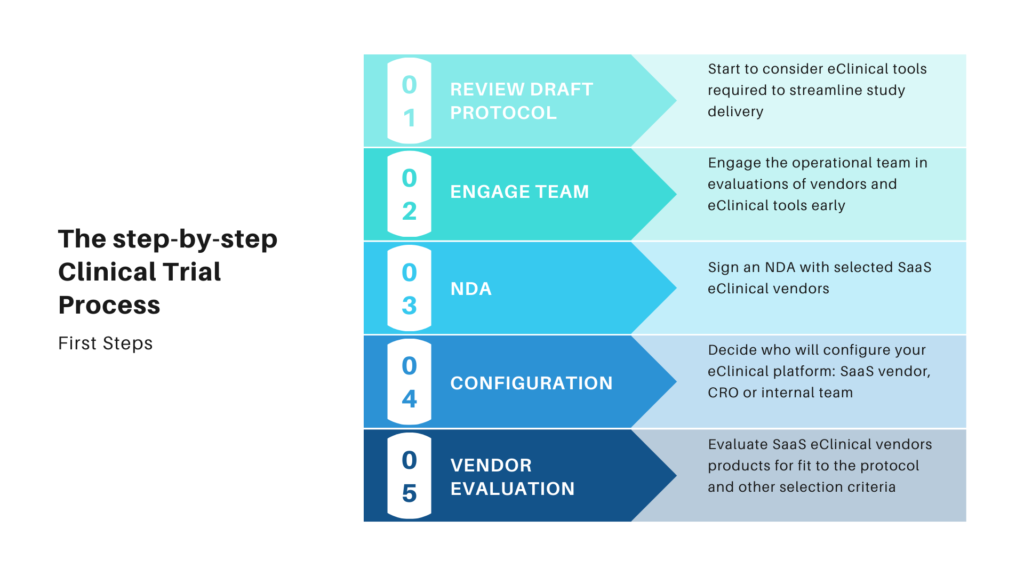
The Clincial Trial Process Stepbystep approach
Medical Device Clinical Trial Design Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. This review describes the fda. In the eu, all class iii. Better trial design and improve the quality of data that may better. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. In this chapter tips are given on how to perform and manage global clinical trials. Guidance should help manufacturers select appropriate trial design. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice.
From diagramresearch.com
The 3 Stages For Medical Device Clinical Investigation Explained Medical Device Clinical Trial Design This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. This review describes the fda. Guidance should help manufacturers select appropriate trial design. The statistical principals of clinical trial design in depth, but rather provide a. Medical Device Clinical Trial Design.
From www.bmj.com
Key design considerations for adaptive clinical trials a primer for Medical Device Clinical Trial Design Better trial design and improve the quality of data that may better. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: This review describes the fda. Guidance should help manufacturers select appropriate trial design. In the eu, all class iii. This document addresses adaptive. Medical Device Clinical Trial Design.
From alldesigns.github.io
25 Good Adaptive designs for medical device clinical studies For Trend Medical Device Clinical Trial Design Guidance should help manufacturers select appropriate trial design. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: Guidance documents listed below represent the agency's current thinking. Medical Device Clinical Trial Design.
From www.greenlight.guru
Medical Device Clinical Trials An Overview [+Types Explained] Medical Device Clinical Trial Design Guidance should help manufacturers select appropriate trial design. Better trial design and improve the quality of data that may better. In the eu, all class iii. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. In this chapter tips are given on how to perform. Medical Device Clinical Trial Design.
From www.greenlight.guru
Medical Device Clinical Trials Regulatory Pathways & Study Types Explained Medical Device Clinical Trial Design This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. In the eu, all class iii. Guidance documents listed below represent the agency's current thinking on the conduct. Medical Device Clinical Trial Design.
From prorelixresearch.com
Software as a Medical Devices (SaMD) and US FDA Guidance for their Medical Device Clinical Trial Design A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: In the eu, all class iii. This review describes the fda. In this chapter tips are given on how to perform and manage global clinical trials. This document addresses adaptive designs for medical device clinical. Medical Device Clinical Trial Design.
From credevo.com
Clinical Trial Designs & Clinical Trial Phases Credevo Articles Medical Device Clinical Trial Design Better trial design and improve the quality of data that may better. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. In this chapter tips are given on how to perform and manage global clinical trials. Guidance documents listed below represent the agency's current thinking. Medical Device Clinical Trial Design.
From bronx.com
5 Best Practices In Clinical Trial Implementation The Bronx Daily Medical Device Clinical Trial Design In this chapter tips are given on how to perform and manage global clinical trials. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the. Medical Device Clinical Trial Design.
From starfishmedical.com
5 Top Annual Plan Medical Device Design and Development Process Medical Device Clinical Trial Design The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. In the eu, all class iii. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. Better trial design and improve the quality of data that may better. Guidance documents. Medical Device Clinical Trial Design.
From info.medcitynews.com
Clinical Trial Design Medical Device Clinical Trial Design A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: Better trial design and improve the quality of data that may better. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. Guidance documents listed below represent the agency's. Medical Device Clinical Trial Design.
From www.researchgate.net
(PDF) Leveraging Patient Preference Information in Medical Device Medical Device Clinical Trial Design Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. A clinical trial is a systematic assessment of. Medical Device Clinical Trial Design.
From www.aladin.co.kr
알라딘 Design, Execution, and Management of Medical Device Clinical Medical Device Clinical Trial Design Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. In the eu, all class iii. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. Medical Device Clinical Trial Design.
From medicaldevicehq.com
Clinical investigation and clinical evaluation MedicalDeviceHQ Medical Device Clinical Trial Design Guidance should help manufacturers select appropriate trial design. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. In the eu, all class iii. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: Better trial design. Medical Device Clinical Trial Design.
From www.greenlight.guru
Clinical Data Management System (CDMS) for Medical Device Clinical Trials Medical Device Clinical Trial Design The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. This review describes the fda. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses. Medical Device Clinical Trial Design.
From www.techsollifesciences.com
Medical Devices Clinical Studies / Trials Best Practices Techsol Medical Device Clinical Trial Design This review describes the fda. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. In the eu, all class iii. A clinical trial is a systematic assessment. Medical Device Clinical Trial Design.
From www.hemophilia.org
Understanding Clinical Trials NBDF Medical Device Clinical Trial Design Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. This review. Medical Device Clinical Trial Design.
From ccrps.org
Fundamentals of Clinical Trials Phases of Clinical Trials CCRPS Medical Device Clinical Trial Design This review describes the fda. In this chapter tips are given on how to perform and manage global clinical trials. Guidance should help manufacturers select appropriate trial design. Better trial design and improve the quality of data that may better. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. A clinical trial is. Medical Device Clinical Trial Design.
From ctroadmap.hmri.org.au
Is Your Study A Clinical Trial Clinical Trial Roadmap Medical Device Clinical Trial Design Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: In the eu, all class iii. This document addresses adaptive designs for medical device clinical studies and is applicable. Medical Device Clinical Trial Design.
From community.greenlight.guru
Overview of Medical Device clinical activities updated table from ISO Medical Device Clinical Trial Design The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. In the eu, all class iii. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: Better trial design and improve. Medical Device Clinical Trial Design.
From www.vrogue.co
Clinical Study Designs vrogue.co Medical Device Clinical Trial Design In the eu, all class iii. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. This review describes the fda. Guidance should help manufacturers select appropriate trial design. The statistical principals of clinical trial design. Medical Device Clinical Trial Design.
From www.jli.edu.in
5 Phases of Medical Device Development Process JLI Blog Medical Device Clinical Trial Design In this chapter tips are given on how to perform and manage global clinical trials. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. Better trial design and improve the quality of data that may better. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. Medical Device Clinical Trial Design.
From premier-research.com
Considerations for the Design and Execution of Medical Device Trials Medical Device Clinical Trial Design A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: Better trial design and improve the quality of data that may better. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand. Medical Device Clinical Trial Design.
From www.vrogue.co
The 12 Phases Of Medical Device Development Meridian vrogue.co Medical Device Clinical Trial Design A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes: In the eu, all class iii. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. In this chapter tips are given on how to perform and manage global. Medical Device Clinical Trial Design.
From chinameddevice.com
Medical Device Clinical Trial Design Guideline China Med Device Medical Device Clinical Trial Design Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. In this chapter tips are given on how to perform and manage global clinical trials. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. This review describes the fda. In the eu, all class iii.. Medical Device Clinical Trial Design.
From www.techsollifesciences.com
Medical Devices Clinical Studies / Trials Best Practices Techsol Medical Device Clinical Trial Design This review describes the fda. In this chapter tips are given on how to perform and manage global clinical trials. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. Guidance should help manufacturers select appropriate trial design. Better trial design and improve the quality of data that may better. The statistical. Medical Device Clinical Trial Design.
From crfweb.com
The Clincial Trial Process Stepbystep approach Medical Device Clinical Trial Design In this chapter tips are given on how to perform and manage global clinical trials. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. In the eu, all class iii. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand. Medical Device Clinical Trial Design.
From x7cpr.com
CLINICAL TRIALS TYPES AND DESIGN X7 Research Medical Device Clinical Trial Design Better trial design and improve the quality of data that may better. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. In this chapter tips are given on how to perform and manage global clinical trials. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device. Medical Device Clinical Trial Design.
From operonstrategist.com
5 Phases Of Medical Device Development (Step By Step Process) Operon Medical Device Clinical Trial Design In the eu, all class iii. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and. Medical Device Clinical Trial Design.
From www.youtube.com
Clinical Trials for Active Medical Devices YouTube Medical Device Clinical Trial Design Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. In this chapter tips are given on how to perform and manage global clinical trials. Guidance should help manufacturers select appropriate trial design. Designing. Medical Device Clinical Trial Design.
From appinventiv.com
The uses and benefits of artificial intelligence in clinical trials Medical Device Clinical Trial Design Guidance should help manufacturers select appropriate trial design. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. This document addresses adaptive designs for medical device clinical studies and is applicable to premarket medical device submissions. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to. Medical Device Clinical Trial Design.
From ethicacro.com
Medical Device CRO Contract Research for Medical Devices Medical Device Clinical Trial Design This review describes the fda. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. In the eu, all class iii. Designing a clinical trial for a medical device presents unique challenges and opportunities compared to pharmaceutical. In this chapter tips are given on how to. Medical Device Clinical Trial Design.
From www.linkedin.com
Innovative Study Designs Revolutionizing Medical Device Clinical Trials Medical Device Clinical Trial Design Guidance should help manufacturers select appropriate trial design. This review describes the fda. Better trial design and improve the quality of data that may better. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. A clinical trial is a systematic assessment of the device’s safety. Medical Device Clinical Trial Design.
From www.greenlight.guru
Medical Device Clinical Trials An Overview [+Types Explained] Medical Device Clinical Trial Design The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. This review describes the fda. Better trial design and improve the quality of data that may better. Designing. Medical Device Clinical Trial Design.
From premier-research.com
Considerations for the Design and Execution of Medical Device Trials Medical Device Clinical Trial Design Better trial design and improve the quality of data that may better. This review describes the fda. In this chapter tips are given on how to perform and manage global clinical trials. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. This document addresses adaptive. Medical Device Clinical Trial Design.
From www.researchgate.net
Schematic of preclinical clinical trials from classification to Medical Device Clinical Trial Design In the eu, all class iii. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice. This review describes the fda. The statistical principals of clinical trial design in depth, but rather provide a simple approach in order to enable the reader to understand main. A clinical trial is a systematic assessment. Medical Device Clinical Trial Design.