Copper (Ii) Oxide React With Ammonia . Copper(ii) ions oxidize iodide ions to molecular iodine, and in the. What is observed when adding ammonia to cu2+ solution, and why does this happen? The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. The reaction of hexaaquacopper(ii) ions with iodide ions. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction by the addition of sulfuric acid. The reaction between copper (ii) ions and aqueous ammonia. A blue solution will form. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). That precipitate dissolves if you add. Ammonia + copper(ii) oxide = copper + dinitrogen + water. Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form.
from www.slideserve.com
A blue solution will form. What is observed when adding ammonia to cu2+ solution, and why does this happen? In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction by the addition of sulfuric acid. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). That precipitate dissolves if you add. The reaction of hexaaquacopper(ii) ions with iodide ions. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form.
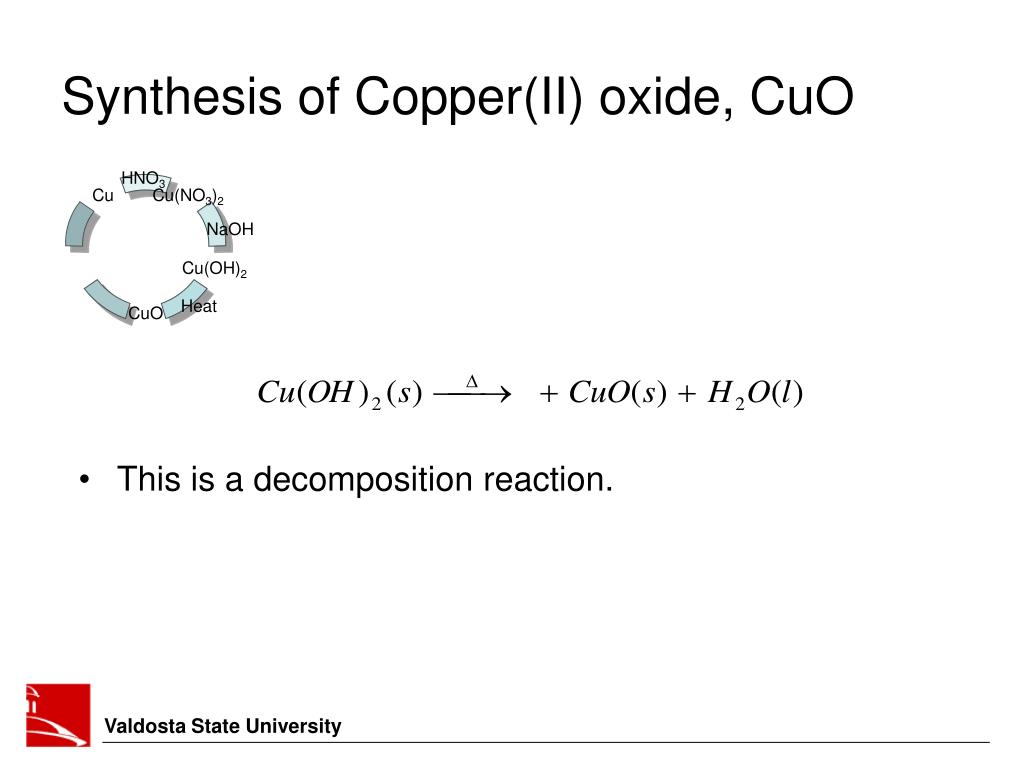
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation
Copper (Ii) Oxide React With Ammonia With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. Ammonia + copper(ii) oxide = copper + dinitrogen + water. A blue solution will form. The reaction between copper (ii) ions and aqueous ammonia. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the. The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. The reaction of hexaaquacopper(ii) ions with iodide ions. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction by the addition of sulfuric acid. Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form. That precipitate dissolves if you add. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). What is observed when adding ammonia to cu2+ solution, and why does this happen?
From www.numerade.com
SOLVED Ammonia gas and solid copper(II) oxide react to form liquid Copper (Ii) Oxide React With Ammonia Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). That precipitate dissolves if you add. A blue solution will form. The limited acid consumption of gangue minerals in ammoniacal systems is. Copper (Ii) Oxide React With Ammonia.
From www.youtube.com
How to Balance Copper (II) sulfate + Ammonium hydroxide YouTube Copper (Ii) Oxide React With Ammonia That precipitate dissolves if you add. The reaction of hexaaquacopper(ii) ions with iodide ions. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place,. Copper (Ii) Oxide React With Ammonia.
From fineartamerica.com
Two Copper Oxide Reactions Photograph by Andrew Lambert Photography Copper (Ii) Oxide React With Ammonia With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. What is observed when adding ammonia to cu2+ solution, and why does this happen? Ammonia + copper(ii) oxide = copper + dinitrogen + water. The reaction between copper (ii) ions and aqueous ammonia.. Copper (Ii) Oxide React With Ammonia.
From www.youtube.com
Copper/Ammonia Coordination Complex and Copper (II) Chloride YouTube Copper (Ii) Oxide React With Ammonia The reaction between copper (ii) ions and aqueous ammonia. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. That precipitate dissolves if you add. What is observed when. Copper (Ii) Oxide React With Ammonia.
From www.youtube.com
Heating a mixture of carbon and copper (II) oxide YouTube Copper (Ii) Oxide React With Ammonia The reaction between copper (ii) ions and aqueous ammonia. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the. Ammonia + copper(ii) oxide = copper + dinitrogen + water. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction by the addition of sulfuric acid.. Copper (Ii) Oxide React With Ammonia.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper (Ii) Oxide React With Ammonia That precipitate dissolves if you add. What is observed when adding ammonia to cu2+ solution, and why does this happen? Ammonia + copper(ii) oxide = copper + dinitrogen + water. The reaction of hexaaquacopper(ii) ions with iodide ions. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo).. Copper (Ii) Oxide React With Ammonia.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper (Ii) Oxide React With Ammonia Copper(ii) ions oxidize iodide ions to molecular iodine, and in the. That precipitate dissolves if you add. The reaction between copper (ii) ions and aqueous ammonia. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light. Copper (Ii) Oxide React With Ammonia.
From www.youtube.com
COPPER AMMONIA COMPLEX FORMATION YouTube Copper (Ii) Oxide React With Ammonia A blue solution will form. The reaction of hexaaquacopper(ii) ions with iodide ions. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). What is observed when adding ammonia to cu2+ solution, and why does this happen? That precipitate dissolves if you add. Two moles of ammonia [nh. Copper (Ii) Oxide React With Ammonia.
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II Copper (Ii) Oxide React With Ammonia Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. What is observed when adding ammonia to cu2+ solution, and why does this happen? Copper(ii) ions oxidize iodide ions to molecular iodine, and in the. Ammonia + copper(ii) oxide = copper + dinitrogen + water. The reaction of hexaaquacopper(ii) ions with iodide ions. Two moles of. Copper (Ii) Oxide React With Ammonia.
From www.numerade.com
SOLVEDWhen ammonia gas is reacted with copper(II) oxide, nitrogen gas Copper (Ii) Oxide React With Ammonia The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the. Ammonia + copper(ii) oxide = copper + dinitrogen. Copper (Ii) Oxide React With Ammonia.
From www.toppr.com
Write balanced chemical equations for the following word equation Copper (Ii) Oxide React With Ammonia That precipitate dissolves if you add. Ammonia + copper(ii) oxide = copper + dinitrogen + water. The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). A blue solution will form. With a small. Copper (Ii) Oxide React With Ammonia.
From www.youtube.com
Copper(ii) Oxide Reduction using Hydrogen YouTube Copper (Ii) Oxide React With Ammonia That precipitate dissolves if you add. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction by the addition of sulfuric acid. Ammonia + copper(ii) oxide = copper + dinitrogen + water. The reaction between copper (ii) ions and aqueous ammonia. Copper(ii) ions oxidize iodide ions. Copper (Ii) Oxide React With Ammonia.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper (Ii) Oxide React With Ammonia The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. A blue solution will form. Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react. Copper (Ii) Oxide React With Ammonia.
From brainly.com
Nitrogen gas can be prepared by passing gaseous ammonia over solid Copper (Ii) Oxide React With Ammonia Copper(ii) ions oxidize iodide ions to molecular iodine, and in the. That precipitate dissolves if you add. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Ammonia + copper(ii) oxide = copper + dinitrogen + water. What is observed when adding ammonia to cu2+ solution, and why does this happen? The coefficients tell you the. Copper (Ii) Oxide React With Ammonia.
From www.chegg.com
Solved Correct PartC olid copper (II) oxide reacts with Copper (Ii) Oxide React With Ammonia Ammonia + copper(ii) oxide = copper + dinitrogen + water. The reaction of hexaaquacopper(ii) ions with iodide ions. What is observed when adding ammonia to cu2+ solution, and why does this happen? A blue solution will form. That precipitate dissolves if you add. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in. Copper (Ii) Oxide React With Ammonia.
From www.youtube.com
Reaction of copper sulfate with ammonia YouTube Copper (Ii) Oxide React With Ammonia That precipitate dissolves if you add. The reaction of hexaaquacopper(ii) ions with iodide ions. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction by the addition of sulfuric acid. What is observed when adding ammonia to cu2+ solution, and why does this happen? Two moles. Copper (Ii) Oxide React With Ammonia.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID7032715 Copper (Ii) Oxide React With Ammonia In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction by the addition of sulfuric acid. That precipitate dissolves if you add. Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form. A blue solution will form. Copper(ii) ion reacts. Copper (Ii) Oxide React With Ammonia.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper (Ii) Oxide React With Ammonia The reaction between copper (ii) ions and aqueous ammonia. A blue solution will form. What is observed when adding ammonia to cu2+ solution, and why does this happen? The reaction of hexaaquacopper(ii) ions with iodide ions. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). The limited. Copper (Ii) Oxide React With Ammonia.
From www.sciencephoto.com
Copper (II) oxide reacting with sulphuric acid Stock Image C052 Copper (Ii) Oxide React With Ammonia The reaction between copper (ii) ions and aqueous ammonia. The reaction of hexaaquacopper(ii) ions with iodide ions. A blue solution will form. The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction. Copper (Ii) Oxide React With Ammonia.
From fineartamerica.com
Copper (ii) Reaction Photograph by Andrew Lambert Photography/science Copper (Ii) Oxide React With Ammonia A blue solution will form. That precipitate dissolves if you add. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the. With a. Copper (Ii) Oxide React With Ammonia.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper (Ii) Oxide React With Ammonia That precipitate dissolves if you add. Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form. The reaction between copper (ii) ions and aqueous ammonia. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. What is. Copper (Ii) Oxide React With Ammonia.
From pixels.com
Copper Sulphate Reacts With Ammonium Photograph by Jerry Mason/science Copper (Ii) Oxide React With Ammonia Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form. The reaction between copper (ii) ions and aqueous ammonia. The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. A blue solution will form. That precipitate. Copper (Ii) Oxide React With Ammonia.
From www.sciencephoto.com
Ammonia reacts with copper sulfate Stock Image C043/4940 Science Copper (Ii) Oxide React With Ammonia What is observed when adding ammonia to cu2+ solution, and why does this happen? With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. Ammonia + copper(ii) oxide = copper + dinitrogen + water. Two moles of ammonia [nh 3] and three moles. Copper (Ii) Oxide React With Ammonia.
From www.researchgate.net
(PDF) Reaction of ammonium chloride with the copper(II) sulfide and Copper (Ii) Oxide React With Ammonia Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction by the addition of sulfuric acid. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3. Copper (Ii) Oxide React With Ammonia.
From www.doubtnut.com
When hydrogen gas is passed over heated copper (II) oxide, copper and Copper (Ii) Oxide React With Ammonia Copper(ii) ions oxidize iodide ions to molecular iodine, and in the. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse. Copper (Ii) Oxide React With Ammonia.
From www.slideserve.com
PPT REACTION PREDICTION PreAP CHEMISTRY PowerPoint Presentation Copper (Ii) Oxide React With Ammonia The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). Ammonia + copper(ii) oxide = copper + dinitrogen + water. The reaction of hexaaquacopper(ii) ions with iodide ions. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion. Copper (Ii) Oxide React With Ammonia.
From www.alamy.com
Copper II ion solutions. Solution of copper II ions (Cu2) in copper Copper (Ii) Oxide React With Ammonia With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. The reaction of. Copper (Ii) Oxide React With Ammonia.
From www.youtube.com
Sodium hydroxide and ammonia added to copper (II) chloride solution Copper (Ii) Oxide React With Ammonia The limited acid consumption of gangue minerals in ammoniacal systems is an added benefit. Ammonia + copper(ii) oxide = copper + dinitrogen + water. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). A blue solution will form. In this experiment, students add ammonia to a solution. Copper (Ii) Oxide React With Ammonia.
From www.numerade.com
SOLVED Consider the reaction of copper(II) oxide with aluminum metal Copper (Ii) Oxide React With Ammonia Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction by the addition of sulfuric acid. Ammonia + copper(ii) oxide = copper + dinitrogen + water. The reaction of hexaaquacopper(ii). Copper (Ii) Oxide React With Ammonia.
From www.coursehero.com
[Solved] Hydrogen gas is reacted with copper ( II ) oxide and the Copper (Ii) Oxide React With Ammonia The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. A blue solution will form. What is observed when adding ammonia to cu2+ solution, and why does this happen? The reaction between copper (ii) ions and aqueous ammonia. The limited acid consumption of gangue minerals in ammoniacal systems. Copper (Ii) Oxide React With Ammonia.
From www.slideserve.com
PPT Mathematics of Chemical Equations PowerPoint Presentation, free Copper (Ii) Oxide React With Ammonia A blue solution will form. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the. Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). The reaction of hexaaquacopper(ii) ions with. Copper (Ii) Oxide React With Ammonia.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper (Ii) Oxide React With Ammonia Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to form. A blue solution will form. Ammonia + copper(ii) oxide = copper + dinitrogen + water. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. What is. Copper (Ii) Oxide React With Ammonia.
From bnrc.springeropen.com
Copper(II) oxide nanocatalyst preparation and characterization green Copper (Ii) Oxide React With Ammonia The reaction between copper (ii) ions and aqueous ammonia. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Two moles of ammonia [nh 3] and three moles of copper(ii) oxide [cuo] react to. Copper (Ii) Oxide React With Ammonia.
From www.mdpi.com
Coatings Free FullText ElectroOxidation of Ammonia over Copper Copper (Ii) Oxide React With Ammonia With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex. The reaction of hexaaquacopper(ii) ions with iodide ions. The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). Two moles of. Copper (Ii) Oxide React With Ammonia.
From www.bartleby.com
Answered How many moles of ammonia would be… bartleby Copper (Ii) Oxide React With Ammonia The coefficients tell you the mole ratios, so 2 moles ammonia (nh3) will react with 3 moles of copper (ii) oxide, (cuo). Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. In this experiment, students add ammonia to a solution of copper (ii) sulfate, observe the colour changes taking place, and then reverse the reaction. Copper (Ii) Oxide React With Ammonia.