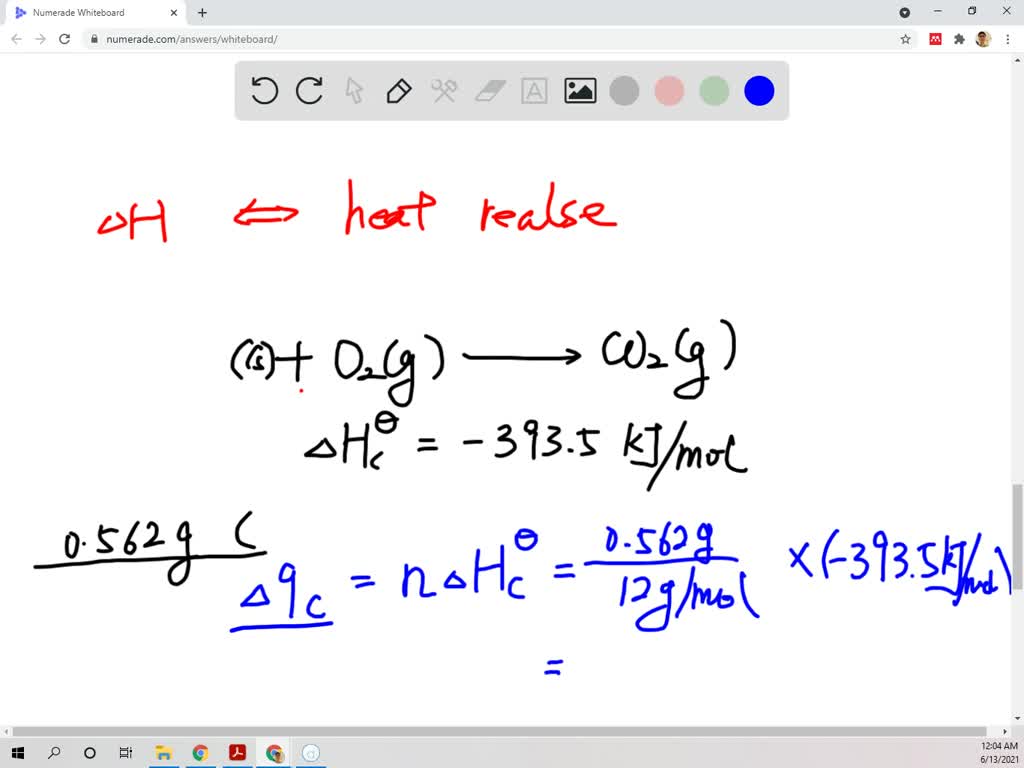
Graphite + Oxygen Equation . This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. The autoignition temperature of graphite is $\pu{730^oc}$. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. H 2 + o 2 = h 2 o however, this. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: The aggregate state is given in parentheses following the formula, such as: This means that pure graphite will ignite at that temperature under.
from solvedlib.com
H 2 + o 2 = h 2 o however, this. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. The aggregate state is given in parentheses following the formula, such as: This means that pure graphite will ignite at that temperature under. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. The autoignition temperature of graphite is $\pu{730^oc}$.
260.562 g of graphite is placed in calorimeter with e… SolvedLib
Graphite + Oxygen Equation For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: H 2 + o 2 = h 2 o however, this. This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. The aggregate state is given in parentheses following the formula, such as: This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. The autoignition temperature of graphite is $\pu{730^oc}$. This means that pure graphite will ignite at that temperature under.
From www.bartleby.com
Answered Diamond and graphite are two… bartleby Graphite + Oxygen Equation H 2 + o 2 = h 2 o however, this. This means that pure graphite will ignite at that temperature under. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is:. Graphite + Oxygen Equation.
From www.toppr.com
Di 0 .0 Ig of graphite is burnt in a bomb calorimeter in excess of Graphite + Oxygen Equation This means that pure graphite will ignite at that temperature under. The aggregate state is given in parentheses following the formula, such as: H 2 + o 2 = h 2 o however, this. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. For example, in the reaction of hydrogen. Graphite + Oxygen Equation.
From byjus.com
51 For the allotropic change represented by the equation C(graphite Graphite + Oxygen Equation H 2 + o 2 = h 2 o however, this. The autoignition temperature of graphite is $\pu{730^oc}$. This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: This article. Graphite + Oxygen Equation.
From www.toppr.com
An organic compound contains C = 36 and H = 6 and rest oxygen. Its Graphite + Oxygen Equation This means that pure graphite will ignite at that temperature under. The aggregate state is given in parentheses following the formula, such as: This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation. Graphite + Oxygen Equation.
From socratic.org
Question b0d55 Socratic Graphite + Oxygen Equation The aggregate state is given in parentheses following the formula, such as: This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. This means that pure graphite will ignite at that temperature under. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation. Graphite + Oxygen Equation.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free Graphite + Oxygen Equation The aggregate state is given in parentheses following the formula, such as: H 2 + o 2 = h 2 o however, this. The autoignition temperature of graphite is $\pu{730^oc}$. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. For example, in the reaction of hydrogen (h₂) with oxygen (o₂). Graphite + Oxygen Equation.
From solvedlib.com
260.562 g of graphite is placed in calorimeter with e… SolvedLib Graphite + Oxygen Equation For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: This means that pure graphite will ignite at that temperature under. This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. H 2 + o 2 = h 2 o. Graphite + Oxygen Equation.
From www.numerade.com
Which of the following chemical equations is connected to the Graphite + Oxygen Equation This means that pure graphite will ignite at that temperature under. H 2 + o 2 = h 2 o however, this. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is:. Graphite + Oxygen Equation.
From brainly.in
Glucose+oxygen=carbon dioxide+water+energy equation Brainly.in Graphite + Oxygen Equation H 2 + o 2 = h 2 o however, this. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: This means that pure graphite will ignite at that temperature under. The aggregate state is given in parentheses following the formula, such as: The autoignition temperature of graphite is $\pu{730^oc}$.. Graphite + Oxygen Equation.
From w20.b2m.cz
1 Mol De Carbono Grafite Reage Com 2 Mols EDUCA Graphite + Oxygen Equation The aggregate state is given in parentheses following the formula, such as: For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. This article reviews the reaction of graphite with. Graphite + Oxygen Equation.
From www.dreamstime.com
The Molecular Formula of Oxygen. Stock Vector Illustration of Graphite + Oxygen Equation This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: This means that pure graphite will ignite at that temperature under. This study is concerned with oxidation steps involving the attachment of. Graphite + Oxygen Equation.
From animal3dwallpaper.pages.dev
Cellular Respiration Formula Equation Graphite + Oxygen Equation The aggregate state is given in parentheses following the formula, such as: This means that pure graphite will ignite at that temperature under. This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. H 2 + o 2 = h 2 o however, this. This article reviews the reaction. Graphite + Oxygen Equation.
From www.tessshebaylo.com
Balanced Equation For The Reaction Between Carbon Dioxide And Water Graphite + Oxygen Equation This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. This means that pure graphite will ignite at that temperature under. The aggregate state is given in parentheses following the formula, such as: This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics,. Graphite + Oxygen Equation.
From colouremployer8.gitlab.io
Casual Carbon Monoxide And Oxygen Equation Parts Of A Balanced Chemical Graphite + Oxygen Equation The aggregate state is given in parentheses following the formula, such as: This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. This means that pure graphite will ignite at that temperature under. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation. Graphite + Oxygen Equation.
From www.alamy.com
The chemical equation with the reactants (methane and oxygen) and the Graphite + Oxygen Equation This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. The aggregate state is given in parentheses following the formula, such as: This means that pure graphite will ignite at that temperature under. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics,. Graphite + Oxygen Equation.
From www.semanticscholar.org
Figure 2 from Composition of Oxygen Functional Groups on Graphite Graphite + Oxygen Equation For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: The aggregate state is given in parentheses following the formula, such as: The autoignition temperature of graphite is $\pu{730^oc}$. This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. This. Graphite + Oxygen Equation.
From ar.inspiredpencil.com
Oxygen Structural Formula Graphite + Oxygen Equation H 2 + o 2 = h 2 o however, this. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: The aggregate state is given in parentheses following the formula, such as: This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport,. Graphite + Oxygen Equation.
From www.numerade.com
SOLVED For the following reaction, 0.158 moles of carbon (graphite Graphite + Oxygen Equation This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. H 2 + o 2 = h 2 o however, this. The aggregate state is given in parentheses following the formula, such as: The autoignition temperature of graphite is $\pu{730^oc}$. This means that pure graphite will ignite at that. Graphite + Oxygen Equation.
From www.youtube.com
How to Balance C + O2 = CO2 (Carbon + Oxygen gas) YouTube Graphite + Oxygen Equation The aggregate state is given in parentheses following the formula, such as: This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: The autoignition temperature of graphite is $\pu{730^oc}$. This. Graphite + Oxygen Equation.
From www.alamy.com
Reaction of Hydrogen and Oxygen in New compounds. Water molecule that Graphite + Oxygen Equation H 2 + o 2 = h 2 o however, this. This means that pure graphite will ignite at that temperature under. The aggregate state is given in parentheses following the formula, such as: This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. For example, in the reaction of hydrogen. Graphite + Oxygen Equation.
From www.numerade.com
SOLVED For the following reaction, 7.40 grams of carbon (graphite) are Graphite + Oxygen Equation This means that pure graphite will ignite at that temperature under. H 2 + o 2 = h 2 o however, this. The aggregate state is given in parentheses following the formula, such as: This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. This article reviews the reaction. Graphite + Oxygen Equation.
From www.alamy.com
Full labelled diagram of the laboratory preparation of oxygen from Graphite + Oxygen Equation The aggregate state is given in parentheses following the formula, such as: The autoignition temperature of graphite is $\pu{730^oc}$. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. For. Graphite + Oxygen Equation.
From stock.adobe.com
Vecteur Stock Photosynthesis equation. Carbon dioxide, water, sugars Graphite + Oxygen Equation This means that pure graphite will ignite at that temperature under. The aggregate state is given in parentheses following the formula, such as: This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation. Graphite + Oxygen Equation.
From www.researchgate.net
Raman spectra of graphite oxide and grapheme oxide. Download Graphite + Oxygen Equation This means that pure graphite will ignite at that temperature under. H 2 + o 2 = h 2 o however, this. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: The autoignition temperature of graphite is $\pu{730^oc}$. This article reviews the reaction of graphite with molecular oxygen in terms. Graphite + Oxygen Equation.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas And Oxygen Gas Reaction Graphite + Oxygen Equation This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. H 2 + o 2 = h 2 o however, this. The aggregate state is given in parentheses following the formula, such as: For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation. Graphite + Oxygen Equation.
From socratic.org
What is the chemical formula of graphite? Socratic Graphite + Oxygen Equation The aggregate state is given in parentheses following the formula, such as: This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. This means that pure graphite will ignite at that temperature under. H 2 + o 2 = h 2 o however, this. This article reviews the reaction. Graphite + Oxygen Equation.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Oxygen Gas Equation Graphite + Oxygen Equation H 2 + o 2 = h 2 o however, this. The aggregate state is given in parentheses following the formula, such as: This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the. Graphite + Oxygen Equation.
From ar.inspiredpencil.com
Structural Formula Of Oxygen Graphite + Oxygen Equation The aggregate state is given in parentheses following the formula, such as: The autoignition temperature of graphite is $\pu{730^oc}$. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. H 2 + o 2 = h 2 o however, this. This means that pure graphite will ignite at that temperature under.. Graphite + Oxygen Equation.
From armsingle10.pythonanywhere.com
Perfect Anaerobic Respiration Reaction Equation Rules Of Balancing Graphite + Oxygen Equation This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: H 2 + o 2 = h 2 o however, this. This means that pure graphite will ignite at that. Graphite + Oxygen Equation.
From www.thesciencehive.co.uk
Group 2* — the science sauce Graphite + Oxygen Equation This means that pure graphite will ignite at that temperature under. The aggregate state is given in parentheses following the formula, such as: H 2 + o 2 = h 2 o however, this. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. The autoignition temperature of graphite is $\pu{730^oc}$.. Graphite + Oxygen Equation.
From www.aquaportail.com
Graphène définition et explications Graphite + Oxygen Equation The aggregate state is given in parentheses following the formula, such as: H 2 + o 2 = h 2 o however, this. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: This means that pure graphite will ignite at that temperature under. This article reviews the reaction of graphite. Graphite + Oxygen Equation.
From jinsuncarbon.com
What is the chemical formula of graphite? Jinsun Carbon Graphite + Oxygen Equation This means that pure graphite will ignite at that temperature under. This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. The aggregate state is given in parentheses following the. Graphite + Oxygen Equation.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between butane Graphite + Oxygen Equation The autoignition temperature of graphite is $\pu{730^oc}$. This study is concerned with oxidation steps involving the attachment of molecular oxygen to the graphene, the formation of carbon monoxide. The aggregate state is given in parentheses following the formula, such as: H 2 + o 2 = h 2 o however, this. This article reviews the reaction of graphite with molecular. Graphite + Oxygen Equation.
From stock.adobe.com
Chemical Equation Infographic diagram showing identities and quantities Graphite + Oxygen Equation This article reviews the reaction of graphite with molecular oxygen in terms of the reaction kinetics, gas transport, and. The autoignition temperature of graphite is $\pu{730^oc}$. For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: This study is concerned with oxidation steps involving the attachment of molecular oxygen to the. Graphite + Oxygen Equation.
From www.youtube.com
1g of graphite is burnt in a bomb calorimeter in excess of oxygen at Graphite + Oxygen Equation For example, in the reaction of hydrogen (h₂) with oxygen (o₂) to form water (h₂o), the chemical equation is: The aggregate state is given in parentheses following the formula, such as: H 2 + o 2 = h 2 o however, this. The autoignition temperature of graphite is $\pu{730^oc}$. This article reviews the reaction of graphite with molecular oxygen in. Graphite + Oxygen Equation.