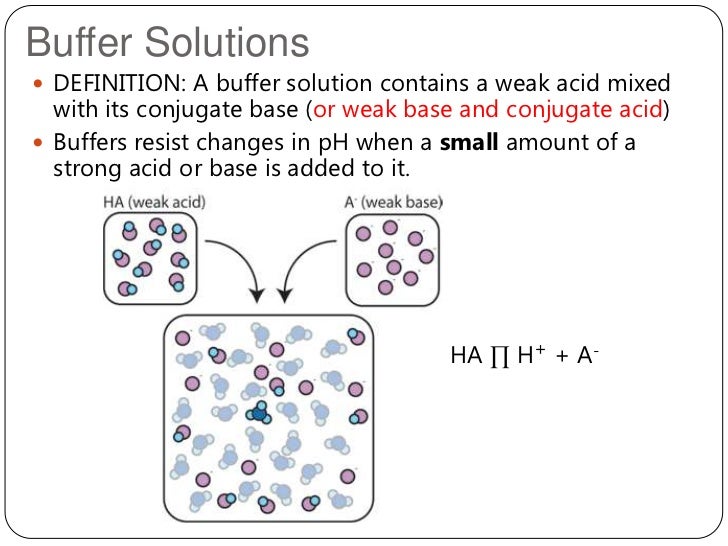
What Happens When You Add A Buffer To An Acid . A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. It is able to neutralize small amounts of added acid or base, thus. Adding a strong electrolyte that contains. This page describes simple acidic and alkaline buffer solutions and explains how they work. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Calculate the ph of a buffer before and after the addition of added acid or base. A solution containing appreciable amounts of a weak. A buffer solution is one which resists changes in ph.
from www.slideshare.net
Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). It is able to neutralize small amounts of added acid or base, thus. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which resists changes in ph. What is a buffer solution? Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Calculate the ph of a buffer before and after the addition of added acid or base. A solution containing appreciable amounts of a weak. Buffers are solutions that resist a change in ph after adding an acid or a base.
2012 topic 18 2 buffer solutions
What Happens When You Add A Buffer To An Acid Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. What is a buffer solution? A solution containing appreciable amounts of a weak. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Adding a strong electrolyte that contains. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Calculate the ph of a buffer before and after the addition of added acid or base. This page describes simple acidic and alkaline buffer solutions and explains how they work. It is able to neutralize small amounts of added acid or base, thus. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph. Buffers are solutions that resist a change in ph after adding an acid or a base.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Happens When You Add A Buffer To An Acid It is able to neutralize small amounts of added acid or base, thus. Adding a strong electrolyte that contains. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Calculate the ph of a buffer before and after the addition of added acid or base. In order for a buffer to. What Happens When You Add A Buffer To An Acid.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download What Happens When You Add A Buffer To An Acid This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? It is able to neutralize small amounts of added acid or base, thus. Adding a strong electrolyte that contains. Calculate the ph. What Happens When You Add A Buffer To An Acid.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Happens When You Add A Buffer To An Acid Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). A buffer solution is one which resists changes in ph. This page describes simple acidic and alkaline buffer solutions and explains how they work. Buffers are solutions that resist a change in ph after adding an acid or a base. Calculate the ph of a buffer before and. What Happens When You Add A Buffer To An Acid.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks What Happens When You Add A Buffer To An Acid Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Calculate the ph of a buffer before and after the addition of added acid or base. A buffer is a solution that can resist ph change. What Happens When You Add A Buffer To An Acid.
From saylordotorg.github.io
Buffers What Happens When You Add A Buffer To An Acid A solution containing appreciable amounts of a weak. Calculate the ph of a buffer before and after the addition of added acid or base. What is a buffer solution? A buffer solution is one which resists changes in ph. It is able to neutralize small amounts of added acid or base, thus. Buffers resist dramatic changes in ph by being. What Happens When You Add A Buffer To An Acid.
From www.youtube.com
The Change in pH with the Addition of a Strong Acid to a Buffer YouTube What Happens When You Add A Buffer To An Acid A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This page describes simple acidic and alkaline buffer solutions and explains how they work. In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. Buffers. What Happens When You Add A Buffer To An Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Happens When You Add A Buffer To An Acid What is a buffer solution? It is able to neutralize small amounts of added acid or base, thus. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer solution is one which resists changes in ph. In order for a buffer to. What Happens When You Add A Buffer To An Acid.
From www.numerade.com
SOLVED Consider the generic dissociation equation_ HA + HzO H3Ot + A What Happens When You Add A Buffer To An Acid What is a buffer solution? Buffers are solutions that resist a change in ph after adding an acid or a base. This page describes simple acidic and alkaline buffer solutions and explains how they work. In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic. What Happens When You Add A Buffer To An Acid.
From www.youtube.com
Find the pH of a Buffer after adding NaOH YouTube What Happens When You Add A Buffer To An Acid In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. A buffer solution is one which resists changes in ph. Calculate the ph of a buffer before and after the addition of added acid or base. What is a buffer solution? Either a weak. What Happens When You Add A Buffer To An Acid.
From dramarnathgiri.blogspot.com
EHSQ (Environment,Health,Safety and Quality) Chemistry of buffers and What Happens When You Add A Buffer To An Acid A solution containing appreciable amounts of a weak. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). This page describes simple acidic and alkaline buffer solutions and explains how they work. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer is a. What Happens When You Add A Buffer To An Acid.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free What Happens When You Add A Buffer To An Acid Adding a strong electrolyte that contains. This page describes simple acidic and alkaline buffer solutions and explains how they work. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. What is a buffer solution? A buffer is a solution that can resist ph change upon the addition. What Happens When You Add A Buffer To An Acid.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk What Happens When You Add A Buffer To An Acid A buffer solution is one which resists changes in ph. It is able to neutralize small amounts of added acid or base, thus. Adding a strong electrolyte that contains. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are solutions that resist a change in ph after adding an. What Happens When You Add A Buffer To An Acid.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts What Happens When You Add A Buffer To An Acid Calculate the ph of a buffer before and after the addition of added acid or base. Buffers are solutions that resist a change in ph after adding an acid or a base. This page describes simple acidic and alkaline buffer solutions and explains how they work. Adding a strong electrolyte that contains. Either a weak acid plus a salt derived. What Happens When You Add A Buffer To An Acid.
From solutionpharmacy.in
Applications Of Buffers Solution Parmacy What Happens When You Add A Buffer To An Acid In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. A solution containing appreciable amounts of a weak. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Buffers are solutions that resist a change in ph after adding an. What Happens When You Add A Buffer To An Acid.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Happens When You Add A Buffer To An Acid A buffer solution is one which resists changes in ph. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A solution containing appreciable amounts of a weak. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). It is able to neutralize small amounts of. What Happens When You Add A Buffer To An Acid.
From treybramirezo.blob.core.windows.net
What Is A Buffer Chemistry at treybramirezo blog What Happens When You Add A Buffer To An Acid Calculate the ph of a buffer before and after the addition of added acid or base. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer solution is one which resists changes in ph. A solution containing appreciable amounts of a weak. In order for a. What Happens When You Add A Buffer To An Acid.
From www.hopaxfc.com
How can Biological Buffer maintain the pH value? Blog Hopax Fine What Happens When You Add A Buffer To An Acid A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. It is able to neutralize small amounts of added acid or base, thus. Calculate the. What Happens When You Add A Buffer To An Acid.
From www.studocu.com
Practice Question 3 Buffers Solution Buffers what happens when we add What Happens When You Add A Buffer To An Acid Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). What is a buffer solution? A buffer solution is one which resists changes in ph. Buffers are solutions that resist a change in ph after adding an acid or a base. It is able to neutralize small amounts of added acid or base, thus. A buffer is a. What Happens When You Add A Buffer To An Acid.
From www.osmosis.org
Making buffer solutions Osmosis What Happens When You Add A Buffer To An Acid Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A solution containing appreciable amounts of a weak. In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. Adding a strong electrolyte. What Happens When You Add A Buffer To An Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Happens When You Add A Buffer To An Acid A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. A solution containing appreciable amounts of a weak. This page describes simple acidic and alkaline. What Happens When You Add A Buffer To An Acid.
From www.expii.com
Buffers — Definition & Overview Expii What Happens When You Add A Buffer To An Acid This page describes simple acidic and alkaline buffer solutions and explains how they work. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Adding a strong electrolyte that contains. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. It is able to neutralize small. What Happens When You Add A Buffer To An Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Happens When You Add A Buffer To An Acid This page describes simple acidic and alkaline buffer solutions and explains how they work. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer solution is one which resists changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Calculate the ph. What Happens When You Add A Buffer To An Acid.
From perso.numericable.fr
The acid in the buffer will neutralize an added alkali and the base in What Happens When You Add A Buffer To An Acid Buffers are solutions that resist a change in ph after adding an acid or a base. It is able to neutralize small amounts of added acid or base, thus. A buffer solution is one which resists changes in ph. A solution containing appreciable amounts of a weak. This page describes simple acidic and alkaline buffer solutions and explains how they. What Happens When You Add A Buffer To An Acid.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Happens When You Add A Buffer To An Acid In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. What is a buffer solution? Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Buffers are solutions that resist a change. What Happens When You Add A Buffer To An Acid.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Happens When You Add A Buffer To An Acid Adding a strong electrolyte that contains. Calculate the ph of a buffer before and after the addition of added acid or base. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer is a solution that can resist. What Happens When You Add A Buffer To An Acid.
From www.numerade.com
SOLVED EOC Problem 7.104 A buffer can be prepared using lactic acid What Happens When You Add A Buffer To An Acid In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. What is a buffer solution? Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer solution is one which resists changes in ph. Buffers resist dramatic. What Happens When You Add A Buffer To An Acid.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Happens When You Add A Buffer To An Acid It is able to neutralize small amounts of added acid or base, thus. In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. Adding a strong electrolyte that contains. Either a weak acid plus a salt derived from that weak acid, or a weak. What Happens When You Add A Buffer To An Acid.
From musicbykatie.com
Does Nh4Oh And Hcl Form Buffer? 28 Most Correct Answers What Happens When You Add A Buffer To An Acid It is able to neutralize small amounts of added acid or base, thus. A solution containing appreciable amounts of a weak. In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. Buffers resist dramatic changes in ph by being composed of certain pairs of. What Happens When You Add A Buffer To An Acid.
From animalia-life.club
Phosphate Buffer System Equation What Happens When You Add A Buffer To An Acid Adding a strong electrolyte that contains. It is able to neutralize small amounts of added acid or base, thus. Calculate the ph of a buffer before and after the addition of added acid or base. A buffer solution is one which resists changes in ph. Buffers are solutions that resist a change in ph after adding an acid or a. What Happens When You Add A Buffer To An Acid.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Happens When You Add A Buffer To An Acid Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A solution containing appreciable amounts of a weak. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. It is able to. What Happens When You Add A Buffer To An Acid.
From jackwestin.com
Buffers 2 Acid Base Equilibria MCAT Content What Happens When You Add A Buffer To An Acid Calculate the ph of a buffer before and after the addition of added acid or base. Adding a strong electrolyte that contains. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A solution containing appreciable amounts of a weak. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). A buffer solution is. What Happens When You Add A Buffer To An Acid.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation What Happens When You Add A Buffer To An Acid Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A solution containing appreciable amounts of a weak. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer is a solution that can resist ph change upon the addition of an acidic. What Happens When You Add A Buffer To An Acid.
From byjus.com
When a small amount of HCL is added to a buffer solution of acetic acid What Happens When You Add A Buffer To An Acid In order for a buffer to resist the effect of adding strong acid or strong base, it must have both an acidic and a basic component. A solution containing appreciable amounts of a weak. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers contain a weak acid (\(ha\)) and. What Happens When You Add A Buffer To An Acid.
From www.youtube.com
What Happens to an Acid or a Base in a Water Solution? Activity 2.9 What Happens When You Add A Buffer To An Acid Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Adding a strong electrolyte that contains. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). It is able to neutralize small amounts of added acid or base, thus. In order for a buffer to resist. What Happens When You Add A Buffer To An Acid.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 What Happens When You Add A Buffer To An Acid A solution containing appreciable amounts of a weak. Calculate the ph of a buffer before and after the addition of added acid or base. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Adding a strong. What Happens When You Add A Buffer To An Acid.